Background and Application[1]
1,4-Benzodiphenol, also known as hydroquinone, is an important chemical raw material. In recent years, domestic demand for it has shown a growing trend, and its consumption area has expanded year by year. 1,4-Benzodiphenol is widely used and is an important raw material, intermediate and auxiliary agent for medicine, pesticides, dyes and rubber. It is mainly used in developers, anthraquinone dyes, azo dyes, rubber antioxidants and monomer resists. Polymerizing agents, food stabilizers and coating antioxidants, petroleum anticoagulants, ammonia synthesis catalysts, etc. At present, the application fields of 1,4-benzenediol are gradually expanding. In addition to its applications in traditional fields, there are also new developments in the fields of chemical fertilizers, water treatment, liquid crystal polymers, etc. In addition, it can also be processed into other fine chemicals. Such as 1,4-diamino leucosome, quinocyanine, etc. In recent years, researchers have focused more on the development and improvement of 1,4-benzenediol production processes and catalysts.
Preparation[2][3][4][5][6][7]
1.1 Traditional method
1.1.1 Reppe method
The Reppe method is an earlier preparation method. The reaction process is roughly as follows, under Ru and Rh catalysts. At a temperature of 100-300°C and a pressure of 100-350 MPa, acetylene reacts with carbon monoxide to generate 1,4-benzenediol. This method has a simple process, but the catalyst required is expensive and difficult to recycle.
1.1.2 Aniline oxidation method
Aniline oxidation method is a traditional synthesis process. It has a history of nearly 80 years. At present, some manufacturers in my country still use this method to produce 1,4-benzenediol. The reaction process of this method is: Step 1. Under acidic conditions in the presence of sulfuric acid, use manganese dioxide or sodium dichromate to oxidize aniline into p-benzoquinone; in the second step, p-benzoquinone is used under acidic conditions. Use iron powder to reduce it to 1,4-benzenediol; step 3. After filtration, crystallization and decolorization, the finished product is obtained. The reaction equation is as follows:

This method is mature, easy to control, and has a high yield, but it generates a large amount of manganese salt, iron salt, and ammonium sulfate waste liquid, has a low recovery rate, pollutes the environment, consumes a lot of raw materials, and has high production equipment costs.
1.1.4 Phenol and acetone method
The reaction process of the production method for preparing 1,4-benzenediol by phenol and acetone method (bisphenol A method) is roughly as follows: Step 1, under hydrochloric acid catalytic conditions, phenol and acetone react to generate bisphenol A; Step 2 , under the action of an alkaline catalyst, bisphenol A is decomposed into phenol and p-isopropylphenol; in the third step, p-isopropylphenol is oxidized into 1,4-diphenol and acetone.
1.1.5 Benzene and propylene method
The process is roughly as follows: the alkylation reaction of benzene and propylene produces para-cumyl and meta-cumyl. Para-cumene is separated and oxidized into dicumyl oxide, which is catalytically decomposed into 1,4-diphenol and acetone.
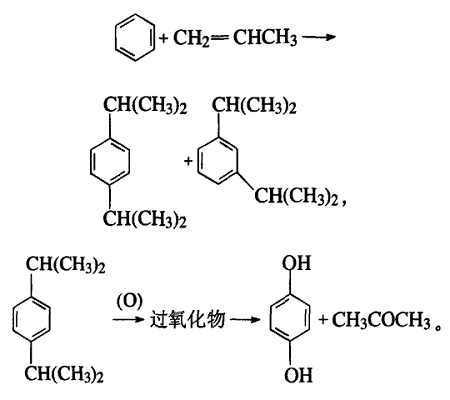
1.2 Cleaning and environmental protection methods
With the development of economy. The requirements for environmental protection are also getting higher and higher. New environmentally friendly processes pose challenges to traditional synthesis processes and are gradually moving towards mature development.
1.2.1 Phenol hydroxylation method – hydrogen peroxide hydroxylation
This method was developed in the 1970s and has now been industrialized. The reaction process is: in the catalystIn the presence of an agent, phenol reacts with hydrogen peroxide to generate 1,4-benzenediol; after dehydration and separation, crude 1,4-benzenediol is obtained; finally, after dissolution, decolorization, and recrystallization, 1,4-benzenediol is obtained. phenol. The reaction equation is as follows:
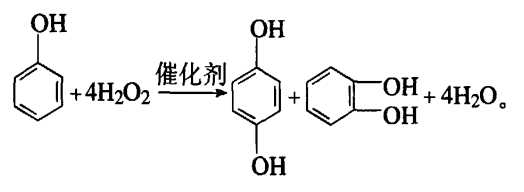
1.2.2 Phenol hydroxylation method – peroxyacid, peroxyketone oxidation method
This law is also called the Ube law, with the successful application of Elben Ube as the first example. The reaction process is: react sulfuric acid, a certain amount of hydrogen peroxide and methyl isobutyl ketone to generate peroxyketone as a catalyst. Catalytically oxidize phenol to produce 1,4-diphenol and catechol: the products are finally separated and purified. The reaction equation is as follows:
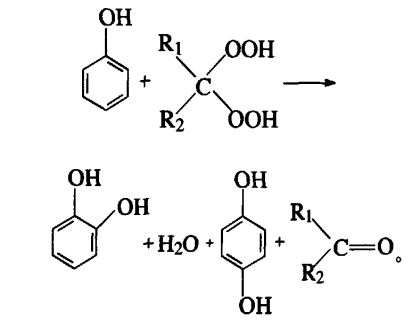
Notes
Routes of invasion: inhalation, ingestion, percutaneous absorption.
Hazards: It is more toxic than phenol and has a strong corrosive effect on skin and mucous membranes. It can inhibit the central nervous system or damage liver and skin functions.
Acute poisoning: Inhalation of high-concentration vapor can cause headache, dizziness, fatigue, blurred vision, pulmonary edema, etc.; accidental ingestion can cause headache, dizziness, tinnitus, pallor, cyanosis, nausea, vomiting, abdominal bloating, and respiratory failure. Difficulty, tachycardia, ulnar syncope, delirium and collapse. In severe cases, hematemesis, hematuria, hemolytic jaundice, and even death.
Chronic effects: Long-term inhalation of low concentrations can cause headache, dizziness, cough, loss of appetite, nausea, vomiting, etc. The skin can cause dermatitis.
Acute toxicity: LD50: 320 mg/kg (orally in rats); oral administration in humans: 5000 mg/kg, death.
Irritation: Human transdermal: 250mg (24 hours), mild irritation.
Subacute and chronic toxicity: Subacute poisoning in animals manifests as hemolytic jaundice, anemia, leukocytosis, increased red blood cell fragility, hypoglycemia, dull coat and obvious cachexia.
Mutagenicity: Microbial mutagenicity: Salmonella typhimurium 2umol/dish. Micronucleus test: human lymphocytes 75umol/L. Sex chromosome deletion and nondisjunction: human lymphocytes 6mg/kg. DNA damage: human bone marrow 500mol/L.
Reproductive toxicity: The lowest oral toxic dose in rats (TDL0): 2500 mg/kg (1 to 22 days of gestation), causing post-implantation mortality (51 days, male), affecting the testicles, epididymis, vas deferens, and prostate , seminal vesicles, etc., which have an impact on male fertility index.
Carcinogenicity: IARC Carcinogenicity Comment: Unclear in animals, no reliable data in humans.
References
[1] Kong Xiangxiang, Fei Shanzai. Technical progress of 1,4-benzenediol production process. Chemical Engineering Times, 1998, 12(1):19-21.
[2] Liu Yingxin, Li Xinxue, Wei Xionghui. Research progress on synthesis methods of 1,4-benzenediol [J]. Chinese Chemical Bulletin, 2004, 67(12): 869-875.
[3] Hu Yucai, Han Dehong, Qi Zenghong, et al. Two-step process for catalytic oxidation of phenol to produce 1,4-benzenediol [J]. Chemical Reaction Engineering and Technology, 2006, 22(6):544 -548.
[4] Rathnam J. Mahalenham, Sunil Ashtkar, Jagadesh Tempi. For the preparation of benzoquinone and 1,4-benzenediol Methods: China, 1918101[P].2007-02-21.
[5] Song Jian, Wang Junbo, Lu Ming, etc. Research on the application of titanium silicon molecular sieve in the synthesis of diphenol [J]. Chemical Industry and Engineering, 2002, 19(2): 159-162.
[6] Yu Jianfeng, Wu Tonghao. Current status of production and research of catechol and 1,4-benzenediol[J]. Fine Petrochemicals, 1997(6): 28-35.
[7] Zhao Yuying, He Muguang, Liu Jingfu. Catalytic activity of heteropoly acids (salts) in phenol hydroxylation reaction[J]. Journal of Northeast Normal University: Natural Science Edition, 1997(2): 4l-44.
Research[J]. Chemical Industry and Engineering, 2002, 19(2): 159-162.
[6] Yu Jianfeng, Wu Tonghao. Current status of production and research of catechol and 1,4-benzenediol[J]. Fine Petrochemicals, 1997(6): 28-35.
[7] Zhao Yuying, He Muguang, Liu Jingfu. Catalytic activity of heteropoly acids (salts) in phenol hydroxylation reaction[J]. Journal of Northeast Normal University: Natural Science Edition, 1997(2): 4l-44.

 微信扫一扫打赏
微信扫一扫打赏

