Background and Overview
4-Fluorophenylboronic acid is a chemical with the chemical formula C6H6BFO2.
Apply [1-3]
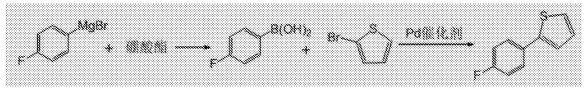
1. Used in the synthesis of 2-(4-fluorophenyl)thiophene
Canagliflozin is a drug for the treatment of diabetes with outstanding clinical performance in the treatment of type 2 diabetes. Among them, 2-(4-fluorophenyl)thiophene is an important intermediate in the synthesis of canagliflozin. 2-(4-Fluorophenyl)thiophene, molecular formula C10H7FS, molecular weight 178.23, is an off-white powdery solid at room temperature. In the existing preparation method of 2-(4-fluorophenyl)thiophene, it is difficult to obtain reaction raw materials , harsh reaction conditions, high energy consumption, high cost, low yield, difficult purification and other shortcomings. In order to overcome the shortcomings and deficiencies of the above-mentioned processes, a new method for synthesizing 2-(4-fluorophenyl)thiophene is needed.
The object of the present invention is to provide a method for synthesizing 2-(4-fluorophenyl)thiophene, which has simple operation process, short production cycle, mild conditions, few side reactions, high yield, and high purity of the resulting product. In order to achieve the above purpose, CN201610223793.2 adopts the following technical solution: a method for synthesizing 2-(4-fluorophenyl)thiophene, including the following steps: 1) Preparation of 4-fluorophenylboronic acid: 4 as material 1 -The fluorophenyl magnesium bromide solution and the borate ester solution as material 2 are respectively pre-insulated to the reaction temperature and then the molar ratio of 4-fluorophenyl magnesium bromide and borate ester is 1:1.0 to 1:2.5. Mix, react at -10°C to 20°C, and then perform quenching and post-treatment to obtain 4-fluorophenylboronic acid; 2) Preparation of 2-(4-fluorophenyl)thiophene: 4-fluorophenylboronic acid Phenylboronic acid, 2-bromothiophene, and Pd catalyst are added to an organic solvent and dissolved to obtain material 3, in which: the molar concentration of 4-fluorophenylboronic acid in the organic solvent is 0.5 mol/L to 2 mol/L, and 4-fluorophenylboronic acid and The molar ratio of 2-bromothiophene is 1:1.0~1:1.5, and the molar ratio of 4-fluorophenylboronic acid and catalyst is 1:0.005~1:0.1; material 3 and the inorganic alkali aqueous solution as material 4 are pre-insulated respectively After reaching the reaction temperature, mix the 4-fluorophenylboronic acid and alkali in a molar ratio of 1:1.0 to 1:3.0, react at 50°C to 80°C, and then perform post-processing to obtain 2-(4- Fluorophenyl)thiophene.
2. Used to prepare a bisfluorosulfone monomer containing a biphenyl structure
Polyaryl ether sulfone (PAES) resin is a type of amorphous thermoplastic special engineering plastic developed in the 1960s. Its molecular backbone consists of a sulfone group, an aromatic ring (phenyl or biphenyl group) and an ether bond. It is known as the first engineering plastic that combines high thermal distortion temperature, high impact strength and excellent processability.
CN201410264880.3 A new bisfluorosulfone monomer containing a biphenyl structure was prepared through molecular design using 4,4-dichlorodiphenylsulfone and p-fluorophenylboronic acid as reaction raw materials, and using the SUZUKI coupling method. , this monomer, like the known bisfluorosulfone monomer, can be widely used in the preparation of high heat-resistant grade biphenyl polyethersulfone resin. The synthesis method is as follows: under nitrogen protection and magnetic stirring, use water and polar organic solvents (1,4-dioxane, N,N-dimethylformamide, N,N -dimethylacetamide or dimethyl sulfoxide) is a mixed solvent, to which 4,4′-dichlorodiphenylsulfone and 4-fluorocarbon are added in a molar ratio of 1:2~5:7~8:0.005~0.1 Phenylboronic acid, anhydrous sodium bicarbonate and palladium catalyst (tetrakis (triphenylphosphine) palladium, bis (tri-tert-butylphosphine) palladium, palladium/carbon, palladium acetate, bis (tricyclohexylphosphine) palladium dichloride etc.), the solid content of the system is 5% ~ 25%, slowly heat up to 60 ~ 90°C, react for 10 ~ 50 hours, cool to room temperature, and discharge the material into deionized water; finally, the obtained product is repeatedly used in deionized water. Rinse until neutral, then rinse with ethanol 3 to 5 times, filter with column chromatography, rotary evaporate, and vacuum dry to obtain a light yellow powder, namely 4,4′-bis(4-fluorophenyl)diphenylsulfone. .
3. Used to prepare a fluorophenylfluorene organic fluorescent material
Among organic fluorescent materials, green light materials have the best performance, followed by red light materials, and blue light materials have the relatively worst luminescent properties, which to a certain extent hinders the development of full-color devices and white light devices. Fluorene, also known as diphenylenemethane, is a polycyclic aromatic hydrocarbon with the molecular formula C13H10. Fluorene has high thermal stability, and the solid film made of it has a certain fluorescence quantum efficiency, and the band gap energy is greater than 2.90eV, but the rigidity of the fluorene structure makes it easy to produce excimers, which affects the luminescence stability of the material, limiting the application of fluorene organic fluorescent materials.
CN201310018100.2 provides an organic fluorescent material with high luminous efficiency and a preparation method with simple steps, mild reaction conditions and high yield. The preparation method of the above-mentioned fluorophenylfluorene organic fluorescent material includes the following steps:
1) Mix 2,7-dibromofluorene, 4-fluorophenylboronic acid, palladium acetate and potassium carbonate in the reaction in a molar ratio of 1~2:2.5~5.0:0.05~0.10:5~10 On device;
2) Prepare a mixed solution with any one of isopropyl alcohol, dimethylformamide and methanol and water at a volume ratio of 4~5:1~2, and then inject the mixed solution into the above reaction device In the air atmosphere, reflux and react at 80~95℃ for 1~2 days;
3) After the reaction is completed, after the reaction solution is cooled, extract it with dichloromethane, combine the organic phases, then wash the organic phase with saturated brine, then dry it with anhydrous magnesium sulfate for 10~12h, filter and obtain the filtrate;
4) Spin dry the organic solvent in the above filtrate to obtain a solid crude product. Use a mixed solvent of petroleum ether and methylene chloride at a volume ratio of 5~6:1~2 as the eluent. The crude product is separated and purified by column chromatography, and petroleum ether and dichloromethane are finally removed to obtain a white powdery solid, which is 2,7-bis(4-fluorophenyl)fluorene.
Main reference materials
[1] CN201610223793.2 A method for synthesizing 2-(4-fluorophenyl)thiophene
[2] CN201410264880.3 A bisfluorosulfone monomer containing a biphenyl structure and its preparation method
[3] CN201310018100.2 A fluorophenylfluorene organic fluorescent material and its preparation method

 微信扫一扫打赏
微信扫一扫打赏

