overview[1]
benzoheptanone can be used as a pharmaceutical synthesis intermediate. recently, the preparation of ketones by introducing a carbonyl group, one of the most important organic groups, into organic compounds has been intensively studied. in one of the most typical methods, aldehydes are reacted with nucleophilic organometallic compounds (e.g., alkylmagnesium halides) to form secondary alcohols, which are then oxidized to ketones such as benzoheptanone with the aid of various oxidants. but this method has several disadvantages. it must go through many reaction steps and produce many unnecessary by-products in the reaction steps. to avoid these problems, active research has been conducted on hydroacylation techniques for the preparation of ketones directly from alkenes and aldehydes by using metal catalysts. a disadvantage of conventional hydroacylation techniques is the tendency to cause side reactions, such as the formation of alkanes from aldehydes via decarbonylation. therefore, in order to suppress this decarbonylation, a technique of introducing carbon monoxide or ethylene gas under high pressure is known, but has the disadvantage of using high-pressure gas and severe reaction conditions.
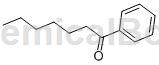
preparation[1]
benzoheptanone is prepared as follows: in a 500 ml pressure reactor, 53 mg of benzaldehyde (0.5 mmol), 11 mg of 2-amino-3-methylpyridine (0.1 mmol), 4 mg of benzoic acid (0.03 mmol), aniline 28 mg (0.3 mmol) and 1-hexene 210 were placed in 80 mg (0.87 mmol) toluene and dissolved in 80 mg (0.87 mmol) toluene. the mixture was stirred at room temperature for 2-3 minutes and then combined with 9.25 mg of rh(pph3)3cl (0.01mmol). while the reactor was stopped with a stopper, the reaction was heated with stirring at 130°c for 1 hour. after the reaction was completed, it was found that phenyheptanone (93 mg, 0.49 mmol) was measured by column chromatography with a yield of 98%.
main reference materials
[1] (us6353139)methodforpreparingketones

 微信扫一扫打赏
微信扫一扫打赏

