Overview[1]
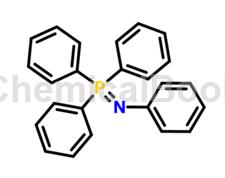
Due to the wide application of heteroatom-containing organic compounds of tetraphenylphosphine in functional materials, biomedicine and other fields, the synthesis of such compounds has received widespread attention. Tetraphenylphosphine has been widely used to synthesize important nitrogen-containing compounds such as guanidines and amidines. At the same time, although N and P belong to the same main group, their bonding properties are very different. For example, N in tetraphenylaminated phosphine is only connected to one benzene ring, while P is connected to three benzene rings at the same time. Considering the difference in electronegativity between N and P, the conjugated extended tetraphenylaminated phosphine derivatives may have special spectral properties. Tetraphenylphosphine can be used as a passivation additive to form a more stable interface film, passivate the surface of the cathode material, make the material structure less likely to collapse, and reduce the decomposition of the electrolyte and lithium salt, thus improving the cycle performance of the battery.
Preparation[2]
(1) Add 19.3g of compound a1(![]() ), add 1L of 5% hydrochloric acid, control the reaction temperature at 5°C, and stir for 1 hour;
), add 1L of 5% hydrochloric acid, control the reaction temperature at 5°C, and stir for 1 hour;
(2) Add 100 mL of 10% sodium nitrite aqueous solution to the above reaction solution, control the reaction temperature at 5°C, and stir for 1 hour;
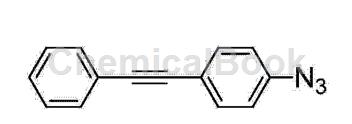
(3) Add 100 mL of 8% sodium azide aqueous solution to the above reaction solution, control the reaction temperature at 5°C, and stir for 1 hour; remove the solvent after extraction, washing, drying, and filtration, and column chromatography will obtain the compound. b1, the compound b1 is the first intermediate of the present invention. The characterization data of the first intermediate are as follows: 1HNMR (500MHz, CDCl3): δ=3.81 (s, 2H), 6.63 (d, J=8.5Hz, 2H), 7.27-7.35 (m, 5H), 7.50 (d , J=8.0Hz, 2H); 13CNMR (125MHz, CDCl3): δ=87.28, 90.06, 112.57, 114.71, 127.63, 128.24, 131.31, 132.93, 146.60;
From the above characterization data, it can be known that the molecular structural formula of the first intermediate is as follows:
(4) Under the protection of inert gas, add 30.4g of compound c1 (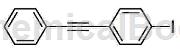 ), add 3L of tetrahydrofuran and 50mL of n-butyllithium (2.5M ), the reaction temperature was controlled at -80°C, and stirred for 2 hours;
), add 3L of tetrahydrofuran and 50mL of n-butyllithium (2.5M ), the reaction temperature was controlled at -80°C, and stirred for 2 hours;
(5) Add 11.1g phosphorus tribromide to the above reaction solution, control the reaction temperature at -25°C, and stir for 2 hours;
(6) Add 40 mL of borane-tetrahydrofuran complex (1M) to the above reaction solution, control the reaction temperature at -25°C, stir for 2 hours, extract, wash, dry, filter, and remove the solvent;
(7) Add 2L diethylamine to the above mixture, control the reaction temperature at 30°C, and stir for 2 hours; remove the solvent after extraction, washing, drying, and filtration, and column chromatography to obtain compound d1, which is The second intermediate of the invention. The characterization data of the second intermediate are as follows: 1HNMR (500MHz, CDCl3): δ=7.29 (t, J=8.0Hz, 6H), 7.35-7.37 (m, 9H), 7.51-7.55 (m, 12H); 13CNMR (125MHz, CDCl3): δ=87.38, 90.11, 122.55 (d, J=7.1Hz), 127.62, 128.36, 128.82,, 132.08, 132.15, 135.88 (d, J=19.6Hz), 136.92 (d, J=10.7 Hz).
(8) Under the protection of inert gas, take 21.9g of compound b1 and 56.3g of compound d1 prepared by the above preparation method, mix them and dissolve them in 5L ether, heat and reflux for 10 hours, and then extract, wash and dry , filter, remove the solvent, and perform column chromatography to obtain the tetraphenylaminated phosphine of this embodiment.
Compound characterization data of tetraphenylphosphine in this method: UV-vis (CH2Cl2) λmax: 316nm; 1HNMR (500MHz, CDCl3): δ=6.77 (d, J=8.0Hz, 2H), 7.21- 7.29(m,5H),7.34(t,J=7.0Hz,9H),7.46(d,J=7.0Hz,2H),7.53-7.55(m,6H),7.60-7.62(m,6H),7.69 -7.74 (m, 6H); 13CNMR (125MHz, CDCl3): δ = 87.61, 88.26 (d, J = 2.1Hz), 90.86, 92.45, 111.56, 122.43, 123.25 (d, J = 18.1Hz), 124.15, 127.26 (d,J=3.1Hz),127.29,128.12,128.38,128.80,129.51(d,J=99.8Hz),131.19,131.69(d,J=12.4Hz),131.72132.33(d,J=10.3Hz) ,132.40,151.43(d,J=2.0Hz);Anal.calcdforC56H36NP:C89.22,H4.81,N1.86;foundC89.13,H4.82,N1.92.
Main reference materials
[1] Fang Jingkun, Yu Xian, & Yang Xin. A tetraphenylaminated phosphine derivative and its synthesis method.
[2] Wan Qiongqiong. (2007). Synthesis and biological activity study of new α-aminophosphonate esters and their amide derivatives. (Doctoral dissertation, Guizhou University).
[3] Wang Ping, Lu Xunfeng, Liu Yong, Lou Yonggang, & Zhang Yuru. A high-temperature electrolyte for lithium-ion batteries.

 微信扫一扫打赏
微信扫一扫打赏

