Background and overview[1]
2-Bromo-4-chloro-3-fluorobenzoic acid is an acid derivative that can be used as a pharmaceutical synthesis intermediate.
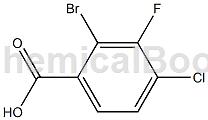
Preparation[1]
2-Bromo-4-chloro-3-fluorobenzoic acid: A solution of 4-chloro-3-fluorobenzoic acid (2.0g, 11.46mmol) in THF (25ml) was passed through a syringe pump at -78°C for 30 minutes. Add to a solution of LDA (13.18 ml, 26.4 mmol) in THF (50 ml). Then stir at -78 °C for 3 h. Then, a solution of 1,2-dibromotetrachloroethane (7.5 g, 23 mmol) in THF (25 ml) was added. The reaction was carried out at -78°C for 30 min, then slowly warmed to room temperature and stirred overnight. The reaction mixture was quenched with water and extracted with Et2O. The aqueous phase was neutralized with 4NHCl in dioxane (45.8 ml, 45.8 mmol) and extracted with EtOAc. The organic phase was dried over MgSO4, filtered and concentrated to give the title compound 2-bromo-4-chloro-3-fluorobenzoic acid. MS(ESI)m/z276.04(M+H).
Apply[1]
2-Bromo-4-chloro-3-fluorobenzoic acid can be used as a pharmaceutical synthesis intermediate, such as preparing 2-bromo-4-chloro-3-fluorobenzaldehyde: BH3.DMS (2.367 ml, 4.73 mmol) was added to a solution of 2-bromo-4-chloro-3-fluorobenzoic acid (1.0 g, 3.95 mmol) in THF (30 ml). After the mixture was stirred at 0 °C for 1 h, the ice bath was removed and the reaction was allowed to proceed at room temperature for 5 h. Additional BH3.DMS (2.367 ml, 4.73 mmol) was added to the reaction mixture at 0°C and stirring was continued overnight while slowly warming to room temperature. The mixture was then treated with 1 N HCl (10 ml) and extracted with EtOAc (2 x 50 ml). The combined organic phases were dried over MgSO4, filtered, concentrated and purified by flash chromatography on silica gel column with 0-30% EtOAc/hexanes to give the title compound (2-bromo-4-chloro-3-fluorophenyl)methanol. PCC (0.57g, 2.66mmol) was added to a solution of (2-bromo-4-chloro-3-fluorophenyl)methanol (0.58g, 2.42mmol) in CH2Cl2 (10ml) at 0°C. Then, the ice bath was removed and the reaction was allowed to proceed at room temperature for 2 h. The solvent was removed and the residue was purified by flash chromatography on silica gel column with 0-20% EtOAc/hexane to give the title compound 2-bromo-4-chloro-3-fluorobenzaldehyde. MS(ESI)m/z238.31(M+H).
Main reference materials
[1] CN201580028124.3 Factor XIa inhibitor

 微信扫一扫打赏
微信扫一扫打赏

