Background and overview[1]
4-n-Butylresorcinol, also known as Rucinol, is an active ingredient in many cosmetics and can inhibit the production of melanin. Specifically, it can inhibit two enzymes necessary for the production of melanin. , namely tyrosinase and 5,6-dihydroxyindole-2-carboxylate-oxidase. There are currently two main methods for synthesizing 4-n-butylresorcinol: Method 1: Using palladium carbon in methanol solvent to reduce 4-butyrylresorcinol through catalytic hydrogenation. Catalytic hydrogenation is a typical gas-liquid-solid three-phase heterogeneous reaction. In order to ensure full contact between the three phases, high temperature and high pressure (15 atm) conditions are required. Not only is the energy consumption high, but the H2 in the system is flammable and explosive. Properties, if leaked, there would be a huge safety hazard, plus the use of palladium carbon, the cost is high. Method 2: Use zinc amalgam to reduce 4-butyrylresorcinol under hydrochloric acid conditions at 120°C. Since the reaction takes place on the surface of activated zinc, a new zinc amalgam needs to be prepared every time this reaction is performed; the reaction temperature is relatively high, resulting in a greater loss of concentration of hydrochloric acid, and concentrated hydrochloric acid needs to be added to maintain the acidity; a large amount of pollution is produced Material zinc amalgam. The two methods mentioned above are conventional methods reported so far, and they have obvious defects during process amplification. In addition, the traditional reactor reaction exotherms and produces a large amount of gas when feeding materials, which poses great safety risks and increases the difficulty of process amplification.
Preparation[1]
4-n-Butylresorcinol is prepared as follows:
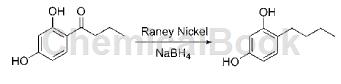
Weigh 100g of 4-n-butyrylresorcinol and dissolve it in toluene, ice-bath it to 0℃, then slowly add 32g (1.5eq) of sodium borohydride in batches, and react at room temperature for 12 hours after the addition is completed; then It is injected into preheating module 1 and preheated to 80°C; 67g of Raney nickel and isopropyl alcohol are mixed and added to preheating module 2 and preheated to 80°C. Then add the aforementioned two preheated materials into the reaction module, and control the residence time at 200s. Collect the flowing out reaction solution, pour it into ice water to quench, filter out the solid, evaporate most of the toluene, add 330 mL of methylene chloride for extraction, evaporate the methylene chloride to dryness, and obtain 82g of 4-n-butylresorcinol as a white solid. The yield was 89%, and the HPLC purity was 99.3%.
Apply[2-4]
4-n-Butylresorcinol inhibits pigment formation by inhibiting the enzyme tyrosinase required for pigment synthesis (pigment production). 4-n-Butylresorcinol first inhibits pigment formation and then prevents the formation of melanin that causes intense coloring of moles. A disadvantage of 4-n-butylresorcinol in formulation is that it tends to discolor itself and the cosmetic or dermatological preparations containing it. Examples of its application are as follows:
1) Prepare a whitening and anti-freckle cream, including a first component, a second component, a third component, a fourth component and a fifth component: the first component includes the following components (in terms of mass percentage Represents): cetearyl alcohol ether-6 and cetearyl alcohol 1.5-2.5; cetearyl alcohol ether-25 1.5-2.5; 16-18 alcohol 1.8-2.8; isooctyl palmitate 2.5-3.5; Squalane 1.5-2.5; the present invention combines the first component, the second component, the third component, the fourth component and the fifth component according to scientific and reasonable proportions to form a whitening and anti-freckle cream, which improves skin absorption. The strength of the whitening and freckle removal cream ensures that the whitening and freckle removal cream can be fully absorbed, and because 4-n-butylresorcinol is a strong antioxidant and a good tyrosine acid reducing agent, it can reduce oxidation and discoloration. The melanocytes are reduced to light color or even colorless, so adding 4-n-butylresorcinol to the whitening and freckle removal cream improves the skin lightening effect of the whitening and freckle removal cream.
2) Prepare a compound resorcinol liposome with high utilization rate and good photostability. The technical solution adopted is: a compound resorcin liposome, which is composed of the following raw materials in mass percentage: phenethyl resorcinol 3%; 4-n-butyl resorcinol 3%, 4-hexyl m-resorcinol. Diphenol 3%, nicotinamide 1%, arbutin α 1%, tranexamic acid 1%, lipid material 5 to 10%; emulsifier 3 to 4%; thickener 0.5 to 2%; light stabilizer 1 ~1.5%; the balance is water; the lipid material is glyceryl neosulfate, the emulsifier is soy lecithin, the thickener is magnesium aluminum silicate, and the light stabilizer is benzyl Acylmethane. The invention has excellent water solubility, high content of active ingredients, is non-toxic and harmless, and has good biocompatibility.
3) Preparation of external skin preparations with substantial whitening cosmetic functions that inhibit excessive melanin formation and thus exhibit skin whitening effects while maintaining natural whitening, including compounds such as the following and/or their salts and 4- n-Butylresorcinol and/or its salt: 5,7-dihydroxy-3,6-dimethoxy-2-(5-hydroxy-4-methoxyphenyl)-4H-l-benzene pyran-4-one, 5,7-dihydroxy-3,6,8-trimethoxy-2-(3,4,5-trihydroxyphenyl)-4H-1-benzopyran-4 -Ketone, 3,5-diethoxy-6,7-dimethoxy-2-(5-ethoxy-4-methoxyphenyl)-4H-l-benzopyran-4- ketone, and 5,6-dihydroxy-3,7-dimethoxy-2-(5-hydroxy-2,4-dimethoxyphenyl)-4H-1-benzopyran-4-one .
Main reference materials
[1] CN201910829650.X A method for synthesizing 4-n-butylresorcinol through microchannel reaction
[2] CN201910753438.X whitening and freckle removal cream
[3] CN201910788257.0 Compound resorcin liposome and preparation method thereof
[4] CN200480037282.7Skin external preparations

 微信扫一扫打赏
微信扫一扫打赏

