Background and overview[1]
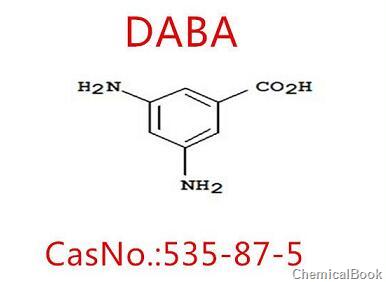
3,5-Diaminobenzoic acid is an important chemical intermediate that can be used to synthesize various pharmaceutical and dye products. For example, a series of yellow reactive dyes derived from it can be used as deep yellow dyes. It can also be used as the yellow component in black dyes, such as reactive orange 6R, which is one of the three primary colors of many reactive blacks and has great market application prospects. 3,5-diaminobenzoic acid is generally obtained by reduction reaction using m-dinitrobenzoic acid as raw material, which mainly includes three methods: iron powder reduction, hydrazine reduction and hydrogenation reduction. The method of producing 3,5-diaminobenzoic acid by reduction of iron powder has lower cost and mature conditions, but the labor protection conditions are poor and the environmental pollution caused by iron sludge is serious.
Apply[1-3]
3,5-Diaminobenzoic acid is an important monomer of the new material polyimide film. The polyimide film is a new type of high-temperature-resistant polymer material with excellent comprehensive properties and is widely used in high-temperature-resistant coatings. , magnet wires, semiconductor protective layers, flexible printed circuit boards, solar modules and other fields, the tough and transparent polyimide film synthesized with 3,5-diaminobenzoic acid as a monomer has excellent performance in maintaining the original polyimide At the same time, it can effectively improve the problem of high-strength and firm bonding between films and other materials.
In addition, 3,5-diaminobenzoic acid is also an important dye and pharmaceutical intermediate. A series of yellow reactive dyes can be derived from it, which can be used as a deep yellow dye or as the yellow component of black dyes. , among which reactive orange 6R is one of the three primary colors of many reactive blacks. It is an important variety of high-end reactive dyes currently encouraged by the country. In the field of medicine, 3,5-diaminobenzoic acid is mainly used to synthesize diatrizoic acid, a contrast agent used for the diagnosis of cardiovascular and cerebrovascular diseases and urinary system diseases; it can also be used to synthesize sulfonamide drugs with broad antibacterial spectrum, low cost and high efficiency. . For example, to prepare an alkoxy side chain polyimide/nano-silica hybrid material: first dissolve 3,5-diaminobenzoic acid-4′-butoxyphenyl ester in N under nitrogen protection. In N-dimethylacetamide, prepare a solution and stir; then add pyromellitic dianhydride in an equal molar amount to the 3,5-diaminobenzoic acid-4′-butoxyphenyl ester into the above solution in three batches. , the polyamic acid solution is obtained by thorough stirring; then the silica sol is added to the polyamic acid solution, and evenly mixed to obtain a mixed glue solution; finally, the mixed glue solution is placed in a vacuum dryer, and then vacuum dried to make it After imidization, an alkoxy side chain polyimide/nano silica hybrid material is obtained. The added amount of the above silica sol is the alkoxy side chain polyimide/nano silica hybrid to be prepared. The amount of silica in chemical materials; it has the advantages of higher thermal stability, mechanical stability and toughness, and can be used in specific technical fields or to modify other general-purpose polyimides.
Preparation[2-4]
Method 1: Preparation method of catalytic hydrogenation of 3,5-diaminobenzoic acid: Prepare an aqueous solution of 3,5-dinitrobenzoic acid with an equal molar equivalent of sodium hydroxide and 5 times the mass of water, and then Under the catalysis of 1% mass homemade Pd/C, the hydrogen pressure during hydrogenation is controlled to 3-4MPa, and the hydrogenation temperature is 50-60 degrees. The reaction reaches a point where the pressure is basically not reduced. After the catalyst is recovered by membrane filtration (directly applied), it is then acidified to PH. value reaches 4-4.5, and after suction filtration and drying, a white to off-white solid is obtained, with a yield of more than 95% and a purity (HPLC) of more than 99%.
Method 2: An industrial continuous hydrogenation method to synthesize 3,5-diaminobenzoic acid, using m-dinitrobenzoic acid and hydrogen as raw materials, which is characterized by including the following steps:
(1) Use water as the reaction solvent, add water and catalyst to a reduction kettle, and add raw materials m-dinitrobenzoic acid and hydrogen respectively to carry out the reduction reaction;
(2) When the reaction liquid in the above-mentioned reduction kettle reaches the liquid outlet, it flows out through the liquid outlet to the second reduction kettle connected in series with the above-mentioned reduction kettle. The unreacted raw materials in the reaction liquid are in the second reduction kettle. Continue the reaction in the reduction kettle;
(3) When the reaction liquid reaches the liquid outlet of the second reduction kettle, it flows out through the liquid outlet to the third reduction kettle connected in series with the second reduction kettle. The unreacted raw materials in the reaction liquid are The reaction continues in the third reduction kettle;
(4) When the reaction liquid reaches the liquid outlet of the third reduction kettle, it flows out through the liquid outlet to the settling equipment for solid-liquid separation, and the resulting filtrate enters the next step;
(5) Adjust the pH of the filtrate in the above step (4) to 4-5. If crystals precipitate, filter the filter cake to obtain 3,5-diaminobenzoic acid.
Main reference materials
[1] CN201610304954.0 A preparation method of 3,5-diaminobenzoic acid
[2] CN201410262664.53, preparation method of catalytic hydrogenation of 5-diaminobenzoic acid
[3] CN201110131175.2 A method for industrialized continuous hydrogenation to synthesize 3,5-diaminobenzoic acid
[4] CN200610039652.1 Preparation method of alkoxy side chain polyimide/nano silica hybrid material

 微信扫一扫打赏
微信扫一扫打赏

