Background and overview[1]
1-Phenyl-2(3-phenylboronic acid)-benzimidazole is an imidazole organic compound that can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
1-Phenyl-2(3-phenylboronic acid)-benzimidazole is prepared as follows:
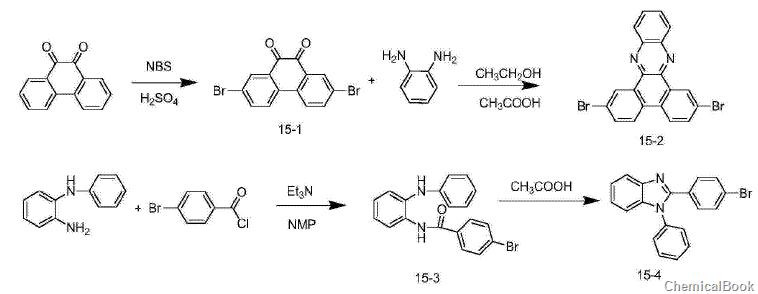
1) Synthesis of compound 15-1: In a three-necked flask, add phenanthrenequinone (6.24g, 30mmol) and concentrated sulfuric acid (50ml), slowly add NBS (11.2g, 63mmol) at 0°C, and react for 2 hours. , slowly pour the reaction solution into ice water, filter, and the obtained solid is recrystallized with dimethyl sulfoxide to obtain 5.6 g of orange solid, with a yield of 50%.
2) Synthesis of compound 15-2: In a three-necked flask, add compound 15-1 (3.66g, 10mmol), o-phenylenediamine (1.2g, 11mmol), acetic acid (40m) and ethanol (80ml), Heated and refluxed for 3 hours, cooled and filtered to obtain 4.2g of light yellow solid with a yield of 96%.
3) Synthesis of compounds 15-3 and 15-4: Add N-phenyl-1,2-phenylenediamine (9.2g, 50mmol) and NMP (80ml, N-methylpyrrolidone) in the flask, Then 4-bromobenzoyl chloride (10.9g, 50mmol) was added and the reaction was stirred at room temperature overnight. After the reaction, pour the reaction solution into water. A large amount of solid will precipitate. Filter. The filter cake is recrystallized with THF (tetrahydrofuran) and methanol. The obtained white solid (compound 15-3) is added to acetic acid (100 ml) and heated to reflux for 12 hours. The reaction proceeds. After completion, the solvent was removed under reduced pressure, methanol (50 ml) was added, and filtered to obtain 12 g of white solid (compound 15-4) with a yield of 69%. Among them, the NMR of 15-4 is:
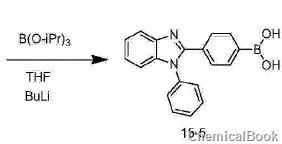
4) Synthesis of compound 15-5 (1-phenyl-2(3-phenylboronic acid)-benzimidazole): Under nitrogen protection, dry compound 15-4 (10.6g, 29mmol) Add tetrahydrofuran (100 mL) into the three-necked flask and cool to -78°C. Then slowly inject 2.5 moles per liter of n-BuLi n-hexane solution (20 mL, 50 mmol) with a syringe under stirring, then add triisopropyl borate (8.1 g, 43 mmol), continue stirring at this temperature for 1 hour, and then Slowly rise to room temperature and stir overnight under nitrogen protection. After the reaction is completed, pour the reaction solution into 2N dilute hydrochloric acid solution and extract three times with ethyl acetate. Combine the organic phases, wash with brine and water in sequence, and dry over anhydrous sodium sulfate. The solvent was removed, and the crude product was recrystallized from ethyl acetate and n-hexane to obtain 8.3 g of white solid 1-phenyl-2(3-phenylboronic acid)-benzimidazole, with a yield of 74%.
Main reference materials
CN201410293118.8 An organic electron transport compound

 微信扫一扫打赏
微信扫一扫打赏

