Background and overview[1][2]
Niraparib p-toluenesulfonate is a sulfonic acid organic compound that can be used as a pharmaceutical intermediate. The chemical name of niraparib is 2-[4-((3S)-3-piperidinyl)phenyl]-2H-indazole-7-formyl. It is an oral polyADP ribose polymer developed by Tesaro Company. Enzyme (PARP) inhibitors can inhibit cells from repairing DNA damage, mainly targeting cancers with BRCA mutations, such as ovarian cancer and breast cancer.
Crystal form[1]
Synthesis method of niraparib p-toluenesulfonate crystal form A: add 120g 2-[4-((3S)-3-piperidyl)phenyl]-2H-indazole-7- into the reaction bottle Formyl p-toluenesulfonate monohydrate, 980 mL of ethanol, 250 mL of water, stir and heat to 80°C under nitrogen protection, dissolve the solid, slowly cool to 20°C while stirring and stir for 2 hours under controlled temperature, filter, and vacuum dry at 40°C to obtain Crystal form A105g.
X-Diffraction Analysis
Test object: the obtained crystal form A;
Detection instrument: Ruiying X-ray diffractometer;
Detection conditions: Cu target Kа ray, voltage 40Kv, current 40mA, 2θ range: 3°-60°, step size 0.02°, dwell time per step 40S;
Testing basis: Pharmacopoeia of the People’s Republic of China 2015 Edition Part 4 0451 X-ray diffraction method;
Test results: Its X-ray powder diffraction pattern contains the following characteristic peaks measured at 2theta angle: 9.5±0.2°, 13.2±0.2°, 15.1±0.2°, 18.4±0.2°, 19.4±0.2°, 21.0± 0.2°, 24.6±0.2° and 30.0±0.2°.
Preparation[2]
The original research company used the following methods to prepare niraparib p-toluenesulfonate:
Route 1 is a synthetic route reported in Journal of Medicinal Chemistry, 2009, Vol. 52, No. 22: In the process of synthesizing niraparib, platinum oxide is used to reduce the pyridine ring. The cost of this reagent is high, among which pyridine The synthesis of the azole ring uses sodium azide. This step is dangerous in scale-up preparation, so avoid using this reagent. After the racemic Niraparib is finally synthesized in this route, a supercritical chiral separation column is used for chiral separation. , the process cost is high and is not conducive to scale-up preparation.

Route 2 Org.ProcessRes.Dev.2011,15,831–840 reports: This synthetic route first obtains (S)-3-(4-aminophenyl)piperidine through chiral resolution, and then combines it with 3- Formyl-2-nitrobenzoic acid methyl ester is condensed, and a pyrazole ring is constructed under the action of sodium azide, and then the Niraparib molecule is synthesized under the conditions of ammonia gas and acid. In this route, the pyridine ring is reduced Platinum oxide is also used, which is relatively expensive. The sodium azide method is also used to construct the pyrazole ring, which is not conducive to amplification.
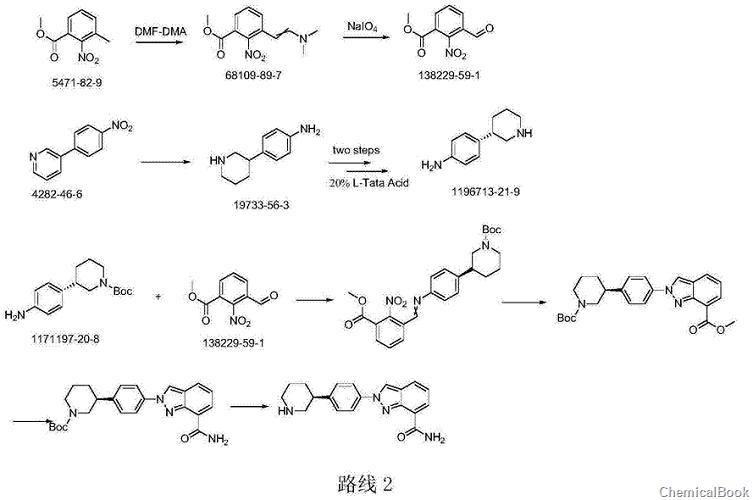
Route 3 Org.ProcessRes.Dev.2014,18,215-227 reports: (S)-3-(4-aminophenyl)ridine is composed of (S)-3-(4-aminophenyl)-2piper (S)-3-(4-aminophenyl)-2-piperidone is obtained by the reduction of sulfidinone, and (S)-3-(4-aminophenyl)-2-piperidone is produced by biological fermentation using specific enzymes. This method hasThere are certain limitations. The selected enzyme needs to be specifically cultured, and the synthesis route is long and costly.
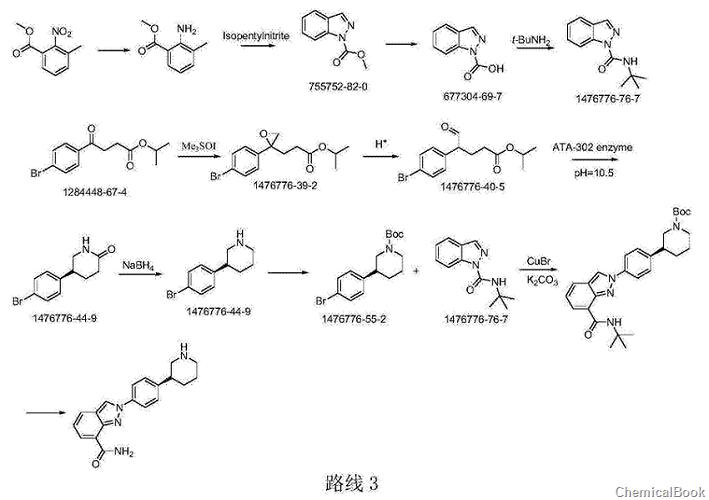
Main reference materials
[1][Chinese invention] CN201810402877.1 A crystal form of niraparib p-toluenesulfonate hydrate and its preparation method
[2]CN201611190679.0 A method for preparing niraparib p-toluenesulfonate monohydrate

 微信扫一扫打赏
微信扫一扫打赏

