Background and overview[1]
3,5-Di-tert-butyl-p-hydroxybenzaldehyde is an aldehyde organic compound that can be used as an intermediate in organic synthesis.
Preparation[1-2]
Method 1: Synthesis of 3,5-di-tert-butyl-4-hydroxybenzaldehyde: Add 2.42mL POCl3 (0.026mol) to 2.01mLDMF (0.026mol) at 0°C, stir until a white solid is produced, add Dissolve in 20 mL of 1,2-dichloroethane to obtain solution A. Dissolve 4.12g of 2,6-di-tert-butylphenol (0.02mol) in 20mL of 1,2-dichloroethane to obtain solution B. Add solution B dropwise to solution A at room temperature, reflux and react at 70°C for 2 hours. After the reaction is completed, add saturated Na2CO3 solution to the reaction solution to neutralize it until no gas is produced, then reflux at 80°C for 40 minutes, separate the liquids, and separate the organic phase. Wash with water, Na2CO3 saturated solution and dry. The organic phase was removed by distillation under reduced pressure, and 4.48g of light brown flaky solid was obtained, with a yield of 96% and a melting point of 185-189°C (literature value 186-190°C);
Method 2: Add 88kg (0.2Kmol) 3.5-di-tert-butyl-4-hydroxytoluene into the reaction pot, then add 300kg methanol, and stir to fully dissolve. Then add 44kg of sodium hydroxide and stir at room temperature for 30 minutes. Add 1.2kg of cobalt chloride and 1.0kg of copper sulfate. Raise the temperature to 60℃ and introduce oxygen at 60-65℃ for 7 hours. Add 320 kg of water to the reaction system, and evaporate the solvent under reduced pressure. The residual liquid was filtered off while hot to remove the catalyst, acidified with hydrochloric acid until the pH value was 3, cooled and filtered with suction to obtain a crude product. Then recrystallize with absolute ethanol and dry to obtain 66.8kg of finished product, with a yield of 70.1% and a product content of 95.18%.
Apply[2]
3,5-di-tert-butyl-p-hydroxybenzaldehyde can be used to prepare (2) 2,4-bis(1-naphthylethyl)-6-(3,5-di-tert-butyl-4-hydroxybenzaldehyde) Ethyl) (B) s-triazine:
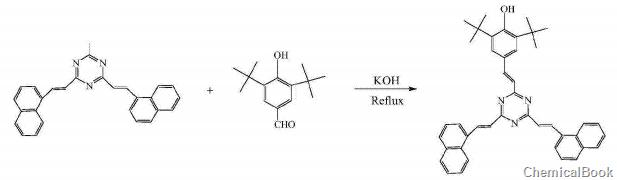
Weigh 1 mole of 3,5-di-tert-butyl-p-hydroxybenzaldehyde and 1.8 mole of potassium hydroxide, then add 20L of methanol to dissolve. Add 5L of 1 molar 1,3,5-trimethyl-s-triazine solution into the flask drop by drop. After the drops are completed, reflux for another 30 hours. Use a rotary evaporator to remove the solvent and the residue with V( Benzene): V (ethanol) = 10:1 toluene-ethanol mixture was used as the eluent, and the yellow powder product was obtained through silica gel column chromatography with a yield of 30.4%, which was confirmed by FTIR, MALDI-TOP, 1HNMR and other methods. The structure of this compound. IR(KBr)ν: 3626, 3063, 3029, 2905, 2026, 1453~1641, 1050, 760~830cm-1; Autoflex III MALDI-TOF mass spectrometer was used to measure its mass spectrum, and its molecular ion peak was 616 (M+H )+. The internal standard substance for NMR measurement was TMS, the solvent was DMSO-D6, and a DRX-400MHz NMR instrument was used. Chemical shift δ: 1.45 (S, 8H), 7.12~7.16 (6H, C=C-H conjugated with the aromatic ring), 7.18~8.44 (m, 16H, hydrogen on the aromatic ring), 9.76 (S, 1H, phenol Hydroxy hydrogen); elemental analysis uses PerkinElmer2400 analyzer, and its molecular formula is C43H41N3O: The theoretical mass percentage of each element is: C83.87; H6.71; N6.82; O2.60; the measured value is C83.69; H6.89; N6 .70; O2.73.
Main reference materials
[1] CN201710094097.0 A preparation method of 3,5-di-tert-butyl-4-hydroxybenzyl alcohol
[2] CN201110318969.X A production process of 3.5-di-tert-butyl-4-hydroxybenzaldehyde

 微信扫一扫打赏
微信扫一扫打赏

