Background and overview[1]
(2R,3S)-3-Benzoylamino-2-hydroxy-3-phenylpropionic acid methyl ester can be passed through 3-hydroxy-4-phenylazetidin-2-one (HPA) It is prepared in two steps and can be used to prepare paclitaxel. Paclitaxel is a mitotic inhibitor or spindle poison and is a new type of anti-microtubule drug.
Preparation[1]
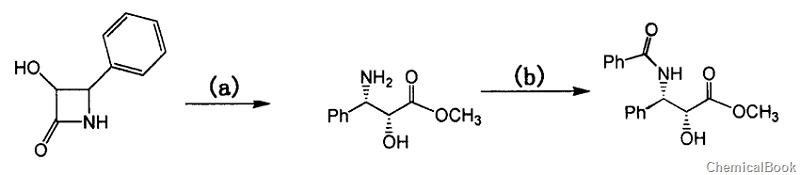
Step a, preparation of 3-phenylisoserine methyl ester hydrochloride
Dissolve 50g of 3-hydroxy-4-phenylazetidin-2-one (HPA) in 300 ml of methanol. Pour HCl gas into the methanol solution of HPA under stirring conditions. After 3 to 4 hours, Ethyl acetate was used as the developing agent for TLC detection. The detection results showed that the raw materials disappeared. The reaction solution was then concentrated under reduced pressure to a dry syrupy product (60g, 100%), which was directly used in the next reaction. 1HNMR (DMSO, 500MHz): δ8.77 (s, 1H), δ7.50 (t, 2H), δ7.41 (m, 3H), δ6.85 (bs, 1H) , δ4.49(d, 1H), δ4.36(s, 1H), δ3.47(s, 3H).MS(m/z): 196(M+1)
Step b, preparation of (2R,3S)-3-benzamido-2-hydroxy-3-phenylpropionic acid methyl ester
Dissolve 3-phenylisoserine methyl ester hydrochloride (obtained in all Example 1) in a mixture of 1000ml CH2Cl2 and 100ml triethylamine (TEA), then add 66ml TEA, cool to -5°C, and start dripping Add 22.6 ml of a solution of benzoyl chloride in dichloromethane (200 ml) (about 30 minutes). After the dropwise addition is completed, stir and react for 2 hours. TLC (ethyl acetate/n-hexane=2/1, v/v) shows that the reaction is complete.
After the reaction is completed, pour the reaction solution into a 3L beaker, wash the reaction bottle with 500ml methylene chloride, put it into the beaker, add 500ml pure water, stir thoroughly, separate the liquids, back-extract the water phase with 250ml methylene chloride, and combine the organic phase, the organic phase was washed with 700 ml of 1N hydrochloric acid, and the liquid was separated. The organic phase was washed with saturated sodium chloride solution 1 (500 ml), the liquid was separated, dried over anhydrous sodium sulfate, and concentrated to dryness.
The crude reaction product was recrystallized in ethyl acetate to obtain a white solid (80g, 89%), mp 165~166°C.
1HNMR (DMSO, 500MHz): δ8.69 (s, 1H), δ8.69 (d, 1H), δ7.83 (d, 2H), δ7.53 (m , 1H), δ7.48(t, 2H), δ7.41(d, 2H), δ7.30(t, 2H), δ7.24(d, 1H), δ5.83(d, 1H), δ5 .42 (dd, 1H), δ4.49 (dd, 1H)., δ3.51 (m, 3H) MS (m/z): 322 (M+Na).
Main reference materials
[1][Chinese invention] CN200710037643.3 isoserine ester derivative and its preparation method

 微信扫一扫打赏
微信扫一扫打赏

