Overview[1]
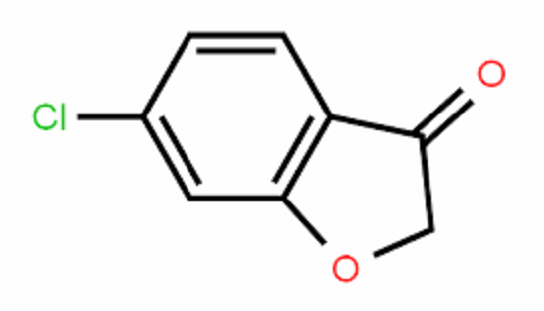
Benzofuranone is an important organic synthesis intermediate and can be used to synthesize medicines, pesticides and new antioxidants. The common synthesis methods are as follows: using o-hydroxyphenylacetic acid as raw material and dehydration, or using o-chlorophenylacetic acid as raw material and hydrolysis and cyclization, or using anisole as raw material to prepare o-hydroxyphenylacetic acid. , obtained by looping again. The raw materials of the former two are not easy to obtain, and the route of the latter is longer.
Apply[3]
Benzofuran-2(3H)-one is an important intermediate in the synthesis of methoxyacrylate fungicide azoxystrobin.
Preparation[2]
1) Put the raw material o-chlorophenylacetic acid into the synthesis kettle, then add the catalyst and liquid caustic soda to the synthesis kettle, and raise the temperature to 100-120°C for reaction;
2) After the reaction in the aforementioned synthesis kettle is completed, the temperature of the synthesis kettle is lowered to room temperature;
3) Filter the materials in the synthesis kettle cooled to room temperature in the aforementioned step 2, and filter and recover the catalyst;
4) Transfer the aforementioned filtered filtrate to the cyclization kettle, put the catalyst and toluene into the cyclization kettle, raise the temperature to 100-120°C, and reflux for 5-8 hours;
5) Add water to the material after the aforementioned reflux to wash the impurities, and transfer the aforementioned organic phase to the concentration kettle for concentration;
6) After concentrating the above to a certain amount of toluene, the toluene solution of benzofuranone is obtained;
7) Distill the concentrated toluene solution of benzofuranone to obtain benzofuranone.
Further, step 1 is to put 800-1000Kg of o-chlorophenylacetic acid as raw material into a 2000L synthesis kettle, then add catalyst and liquid caustic soda to the synthesis kettle, and heat it to 100-120°C for reaction.
Further, step 4 is to transfer the aforementioned filtered filtrate to the cyclization kettle, put the catalyst and 300-500Kg toluene into the cyclization kettle, raise the temperature to 100-120°C, and conduct a reflux reaction for 5-8 hours.
Further, step 5 is to add 150-200Kg of water to the above-mentioned material after the reflux is completed to wash the impurities therein, and transfer the above-mentioned organic phase to the concentration kettle for concentration.
Further, the concentration of liquid caustic soda is 40-42%.
Further, the catalyst in step 1 is one of sodium ethoxide or quaternary ammonium salt.
Furthermore, the catalyst in step 4 is RaneyNi.
The technical characteristics and effects of this method are: compared with the existing technology during operation, the above process overcomes the shortcoming of a long production process route for the azoxystrobin intermediate benzofuranone, and at the same time inherits the existing technology The advantages of the invention are that the raw materials are cheap and easy to obtain, and the operation is simple. Therefore, the present invention reduces the cost of raw materials, the production process is relatively simple to operate, and it is more environmentally friendly.
Main reference materials
[1] Dong Ping, Xi Xiaoli, & Qi Panlun. (2006). Stabilization mechanism of 3-aryl benzofuranone and its role in bopp stabilization process. Progress in Fine Petrochemicals (9), 32-34.
[2] Meng Xin, Xin Zhong, & Cai Zhi. . The role of benzofuranone in inhibiting the degradation process of polypropylene. China Plastics (6), 81-85.
[3] Meng Xin, Zhou Changlu, & Xin Zhong. (2009). Effect of the structure of benzofuranone derivatives on their dpph capture ability. Applied Chemistry, 26(12), 1409-1413.

 微信扫一扫打赏
微信扫一扫打赏

