Background[1]
4-Ethynylbenzaldehyde is an organic synthesis intermediate and pharmaceutical intermediate. It can be used in organic synthesis reactions and pharmaceutical research and development processes in laboratory research and development.
Preparation[1-2]
Method 1: 4-ethynylbenzaldehyde is prepared as follows:
Step 1: In the DMSO solvent system, react carbazole, hexane bromide and 50% sodium hydroxide aqueous solution at a molar ratio of 1:2:2 at 50°C for 8 hours. First, distill under reduced pressure, add water and ethyl acetate. Extract, take the organic layer, then dry it with anhydrous Na2SO4, spin it to dryness, separate and purify to obtain compound 1.
Step 2: react compound 1 and NBS in a dichloromethane solvent system at a molar ratio of 1.2:1 at 25°C for 8 hours, filter, and wash with dichloromethane several times to obtain compound 2.
Step 3: In a dry diisopropylamine solution system under nitrogen protection, add p-bromobenzaldehyde, trimethylethynylsilane, cuprous iodide and tetrakis triphenylphosphorus palladium in molar ratios 1:3: (0.03-0.05): (0.05-0.1) React in a closed state at 80°C for 8 hours, extract with water and ethyl acetate, take the organic layer, dry it with anhydrous Na2SO4, spin it to dryness, separate and purify to obtain the corresponding 4- Add (methylethynyl)benzaldehyde into a 100 mL single-neck bottle, then add 3 equivalents of potassium carbonate, stir for 2 hours, extract, and spin dry to obtain compound 3, whose structure is 4-ethynylbenzaldehyde.
Method 2: 4-ethynylbenzaldehyde is prepared as follows:
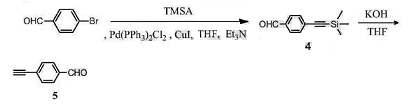
(1) Synthesis of compound 4
Dissolve p-bromobenzaldehyde (1.85g, 10mmol) in 15mL of anhydrous THF, add trimethylsilyl acetylene (1.47g, 15mmol), bistriphenylphosphine palladium dichloride (70mg, 0.1 mmol), copper iodide (38 mg, 0.2 mmol), and 5 ml triethylamine. React at 40°C for 2 hours. Spin THF to dryness and perform column purification using PE and DCM (volume ratio 2:1) as eluents to obtain 2.0 g of compound 4 as a white solid. The yield is 99%.
(2) Synthesis of compound 5
Dissolve compound 4 (727 mg, 3.6 mmol) in 15 mL of anhydrous THF, add 2 mL of KOH aqueous solution (298 mg, 4 mmol), and react at room temperature for 2 h. The THF was spun dry, the reaction solution was extracted with EA, and washed three times with saturated NaCl solution. The organic phase was dried over anhydrous Na2SO4, filtered, concentrated, and purified through column using PE and DCM as eluents (volume ratio 1:1) to obtain 450 mg of compound 5 as a light yellow solid. The yield is 97%.
Main reference materials
[1] CN201711293742.8 Preparation method and application of terpyridine compound and its metal complex
[2] CN201610312523.9 Application of polymer microspheres in Raman detection

 微信扫一扫打赏
微信扫一扫打赏

