Background[1]
4-Fluoro-2-methoxy-5-nitroaniline is an organic synthesis intermediate and pharmaceutical intermediate, which can be used in laboratory research and development processes and chemical and pharmaceutical research and development processes.
Preparation[1-2]
Method 1: 4-fluoro-2-methoxy-5-nitroaniline is prepared as follows:
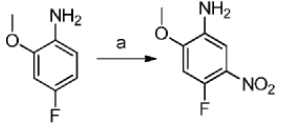
Dissolve 4-fluoro-2-methoxyaniline (20g) in concentrated sulfuric acid at -15°C to dissolve all the solids in the mixture, and slowly add potassium nitrate solution (5.91g) dissolved in concentrated sulfuric acid. Continue stirring at this temperature for 2 hours. Pour the reaction solution into ice water, add NaOH, adjust the pH to 8.0-9.0, stir vigorously to precipitate the solid, and filter it with suction to obtain 4-fluoro-2-methoxy-5-nitroaniline (22.0g) as a yellow solid. The rate is 83.7%.
Method 2: 4-fluoro-2-methoxy-5-nitroaniline is prepared as follows:
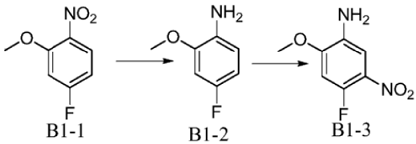
Synthesis of B1-2: Weigh 50g of raw material B1-1, add 500ml of methanol to completely dissolve it, add 10g of Pd/C, and perform hydrogenation reaction at 35°C for two days. Point board monitoring, raw material reaction is completed and processed. Pd/C was directly filtered off, and the methanol phase was spin-dried to obtain 39 g of crude product, which was directly added to the next step.
Synthesis of B1-3: Take 39g of raw material B1-2, add it to 500ml of concentrated sulfuric acid, and add it under an ice-salt bath. Control the temperature below 10°C and stir to completely dissolve. Keep the temperature below 10°C and add 1ep of potassium nitrate and stir at room temperature overnight. The next day, pour into ice water, adjust pH>7 with ammonia, extract with ethyl acetate, dry, and pass through column to obtain 44g of product.
1H-NMR (CDCl3) δ7.39 (d, J=7.2Hz, 1H), 6.63 (d, J=12.4Hz, 1H), 3.94 (s, 3H), 3.90 (broad, 2H).
Apply[1]
4-Fluoro-2-methoxy-5-nitroaniline can be used to prepare the following compounds:
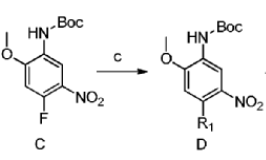
Dissolve 4-fluoro-2-methoxy-5-nitroaniline (22.0g,) triethylamine and DMAP in THF at room temperature, then add Boc2O THF solution dropwise to the reaction system, continue Stir the reaction for 24h. After the reaction is completed, pour into water and extract with dichloromethane three times. The organic layer is washed with water and saturated brine in sequence, then dried over anhydrous sodium sulfate, and evaporated to dryness to obtain a tan solid.
Main reference materials
[1] CN110078732-Purine compounds and their uses
[2] CN108558835-A crystal form, preparation method and use of deuterated AZD9291

 微信扫一扫打赏
微信扫一扫打赏

