Overview[1]
Fluorophenylacetic acid is an important intermediate and plays an irreplaceable role in the synthesis of pharmaceuticals, electronic materials, etc. For example, m-fluorophenylacetic acid can be used in the preparation of anti-inflammatory drugs. For another example, 2,4,5-fluorophenylacetic acid is a key intermediate for the preparation of sitagliptin, a diabetes treatment drug. For another example, 2,3-difluorophenylacetic acid can be used to prepare difluoronaphthol-based liquid crystal intermediates.
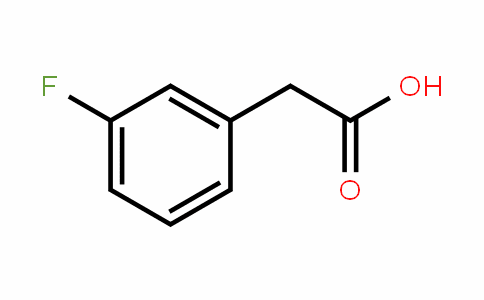
Preparation[1]
Preparation of m-fluorophenyl acetic acid: Add 350 grams of 32% HCl and 250 grams of 3-fluoroaniline into the reaction bottle, heat and stir until dissolved. Cool to -5~5℃, add 20g tetramethylammonium chloride and 20g copper chloride. Add dropwise an acetone solution containing 300 g of vinylidene chloride. Maintaining the temperature, slowly add a solution containing 230g isopropyl nitrite and 200g acetone dropwise. The reaction was kept warm for 2 hours. The reaction is completed and quenched. Dichloromethane was added for extraction and then distilled to obtain 398 g of 1-(2,2,2-trichloroethyl)-3-fluorobenzene. Add 500g of water and 60g of p-toluenesulfonic acid to the three-necked flask under nitrogen protection. Heat to 80~95℃. 300g of intermediate 1-(2,2,2-trichloroethyl)-3-fluorobenzene was added dropwise. After the dropwise addition is completed, the reaction is kept warm for 8 hours. The reaction is complete. The cooling system was added dropwise to ice water to quench. Filter, wash with water, dry and recrystallize with dichloroethane to obtain 151.2g of pure m-fluorophenyl acetic acid, with HPLC purity greater than 99wt%.
Apply[2]
M-fluorophenylacetic acid can be used to prepare m-fluorophenylacetyl chloride: Add m-fluorophenylacetic acid, thionyl chloride and dimethylformamide in sequence to a clean three-necked flask, stir and heat to reflux, the temperature is 110°C. React for 3 to 5 hours. After the reaction is completed, the thionyl chloride is removed by distillation under normal pressure, and the burgundy product at 64°C/5mmHg is collected to obtain m-fluorophenyl acetyl chloride.
Main reference materials
[1] CN201710170399.1 Preparation method of fluorophenylacetic acid
[2] CN201410364152.X A kind of 4-(3-fluorophenyl)-2,2-dimethyl-5-(4-(methylthio)phenyl)furan-3(2H)-one Preparation method

 微信扫一扫打赏
微信扫一扫打赏

