Overview[1][2]
Phenyl pericarboxylic acid, also called 8-aniline-1-naphthalenesulfonic acid, is a dye intermediate used to produce weakly acidic black BR, weakly acidic deep blue 5R, sulfur dyes and azo dyes, and fluorescent detectors for protein research. . Phenyl periacid is a fluorescent dye with high affinity for the hydrophobic surfaces of proteins. Phenyl periacids undergo a blue shift and a significant increase in fluorescence intensity upon binding to protein surfaces in regions of low polarity 1,2. These properties make phenyl periacids well suited for determining the affinity of hydrophobic ligands and their corresponding binding proteins.
Degradation method[1]
Phenyl pericarboxylic acid is an important organic chemical raw material, and its sulfonic acid group is highly toxic to microorganisms.
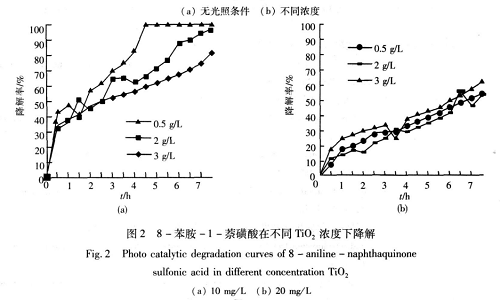
It is harmful to humans and livestock to a certain extent. The mother liquor must be treated before discharge to reduce its sulfonic acid resistance content. Jing Xiuyan and others used TiO2 as a photocatalyst to photocatalytically degrade phenyl periacids in water. The adsorption equilibrium time and adsorption amount of TiO2 to phenyl peri-acid, the dosage of TiO2, the concentration of phenyl peri-acid, the pH value of the solution and the illumination time were discussed. and other factors on the degradation effect. The test results show that under no light conditions, TiO2 has almost no catalytic effect on phenyl perisite acid, but mainly surface adsorption, and the surface adsorption reaches equilibrium in about 30 minutes; UV lamp During irradiation, the reaction time of 10 mg/L phenyl perisite acid solution is 5~7 h, the dosage of TiO2 photocatalyst is 2.5~3 g/L, and the pH is 4~6 When, it is more appropriate, the maximum degradation rate occurs, and the degradation rate can reach more than 95%.
Apply[2]
CN201811260602.5 reports a glycocholic acid detection kit, and provides a preparation method for glycocholic acid-protein conjugates and anti-glycholic acid antibody-coated latex particles in the glycocholic acid detection kit. The glycocholic acid detection kit adopts a competition method to detect glycocholic acid. By adding phenyl peri-acid to the kit, the analytical sensitivity and precision of the kit are significantly improved, and the linear range of the detection is expanded. Moreover, this kit The specific substances have simple preparation steps and low cost, and are more suitable for industrial production and clinical applications.
Main reference materials
[1] Jing Xiuyan, Yang Fan, Tian Pingan. TiO_2 photocatalytic degradation of 8-aniline-1-naphthalenesulfonic acid [J]. Journal of Xi’an University of Science and Technology, 2009, 29(02): 227-230+239.
[2] CN201811260602.5 A glycocholic acid detection kit

 微信扫一扫打赏
微信扫一扫打赏

