Background and overview[1]
4-Bromo-2,3-difluorobenzaldehyde is an important fluorine-containing benzene liquid crystal intermediate. Because the fluorine atom in its structure replaces the hydrogen atom on the benzene ring, relative to other groups, It is close to hydrogen atoms in volume and will not affect the orderly arrangement of liquid crystals due to steric hindrance. At the same time, fluorine atoms have high electronegativity, which can ensure that the fluorine-containing liquid crystal structure still has a certain dipole moment. In addition, the fat solubility of fluorine In addition, compounds containing fluorine in terminals and side chains can significantly increase the solubility of other liquid crystal components in mixed liquid crystal formulas. Fluorine-containing liquid crystals have become the mainstream of thin film transistor liquid crystal displays. Therefore, 4-bromo-2,3-difluorobenzaldehyde is particularly suitable for use as a liquid crystal intermediate and is of great significance for the production of high-performance liquid crystal materials.
Preparation[1]
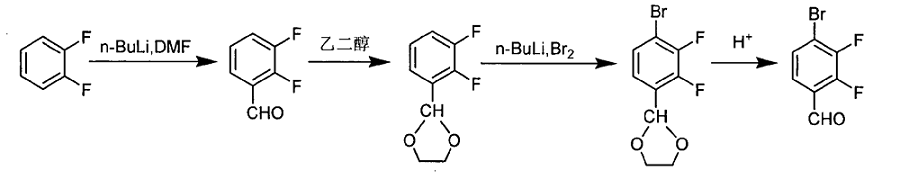
1) Take 1000g of o-difluorobenzene and 5000mL of tetrahydrofuran and add it to a 10L three-necked flask. Replace it with nitrogen three times. When the temperature reaches -65℃, start adding 4000mL of n-butyllithium (2.5M/L) dropwise within 2h. After the dripping is completed and the temperature is maintained for 0.5h, 800g of dimethylformamide is added dropwise and the dripping is completed within 1h. After the dripping is completed, the temperature is naturally raised to -30°C. Pour the reaction solution into 10% dilute hydrochloric acid and control the temperature at 30°C. After washing with water and distilling to remove the solvent, the intermediate 2,3-difluorobenzaldehyde is obtained;
2) Take 1250g of the intermediate 2,3-difluorobenzaldehyde, 800g of ethylene glycol, 3500mL of toluene, and 15g of p-toluenesulfonic acid and add them to the reflux water separation device for reaction. Stop the reaction when no water comes out, and wash and distill it away. After dissolving, the intermediate 2,3-difluorobenzaldehyde ethylene glycol acetate is obtained;
3) Take 1000g of the intermediate 2,3-difluorobenzaldehyde ethylene glycol, 5000mL of tetrahydrofuran, replace it with nitrogen three times, and when the temperature drops to 65℃, start adding 2500mL of n-butyllithium (2.5M/L ), finish dripping within 2 hours. After 0.5 hours of heat preservation, start dripping 1000g of liquid bromine and finish dripping within 1 hour. After completion, naturally raise the temperature to -30°C, pour into water to quench, and add an appropriate amount of sodium thiosulfate to the KI test paper No color develops. After 10 minutes of reaction, the intermediate 4-bromo-2,3-difluorobenzaldehyde ethylene glycol is obtained after washing with water and distilling to remove the solvent;
4) Take the intermediate 4-bromo-2,3-difluorobenzaldehyde ethylene glycol, react with 20% dilute sulfuric acid for 11-13 hours, add an appropriate amount of chloroform to the organic layer to dilute it, wash with water and recrystallize The target product 4-bromo-2,3-difluorobenzaldehyde was obtained.
Main reference materials
[1] [Chinese invention] CN201210134557.5 A new type of liquid crystal intermediate 4-bromo-2,3-difluorobenzaldehyde

 微信扫一扫打赏
微信扫一扫打赏

