Background and overview[1]
Aromatic nitrile compound phthalonitrile is a very important organic synthesis intermediate, widely found in medicines, pesticides, herbicides, insecticides, dyes, spices and natural products. For example, 2,4-dinitro-6-cyanoaniline, which can be used to make excellent bright blue diazo dyes, and the international new nitrile spice lemon nitrile, which has a sharp and strong lemon-like aroma. In addition, the cyano group is a type of functional group containing a carbon-nitrogen triple bond. It has a short bond length, a relatively small volume (about 1/8 of the methyl group), strong polarity and strong electron-withdrawing properties. Therefore, The cyano group is a good hydrogen bond acceptor, allowing it to penetrate deep into the target protein and form strong hydrogen bond interactions with key amino acids in the active site; at the same time, the cyano group is also used in activity studies due to its Metabolic stability and bioisosteres as functional groups such as hydroxyl and carboxyl groups can enhance the interaction between small drug molecules and target proteins, and are therefore widely used in medicinal chemistry research.
Preparation[1]
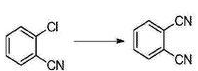
The preparation of phthalonitrile is as follows: under argon protection, add NiCl2·6H2O (0.05mmol, 11.9mg), dppf (0.06mmol, 33.3mg), Zn (0.2mmol, 13.0mg), DMAP (1.0mmol, 122.2mg), Zn(CN)2 (0.8mmol, 93.9mg), p-chloroanisole (1.0mmol, 140.6mg) and 2-chlorobenzonitrile (5.0mL), Then put it directly into a 60°C oil bath for reaction. Stop heating after 6 hours and cool to room temperature. Filter the reaction solution directly through a short silica gel column, rinse with dichloromethane, concentrate, and directly purify by silica gel column chromatography (in view of the fact that the product Most of it is easily pumped away. To avoid sample mixing loss, unless otherwise specified, all samples are loaded by wet method.) Eluent: petroleum ether/ethyl acetate = 3:1, the product is white solid phthalonitrile. 106.1mg, yield 83%, 1HNMR purity greater than 98%. 1HNMR (400MHz, CDCl3, Me4Si): δ7.76-7.80 (m, 2H), 7.81-7.86 (m, 2H). 13CNMR (100MHz, CDCl3, Me4Si): δ115.32, 115.57, 133.23, 133.47.
Main reference materials
[1]CN201810819052.X A method for co-producing methylbenzoic acid, methylbenzonitrile and phthalonitrile

 微信扫一扫打赏
微信扫一扫打赏

