Overview[1]
Dibenzo-18-crown ether-6 is the synthetic raw material for diaminodibenzo-18-crown ether-6, which is an important crown ether compound. , is also an important intermediate raw material for the synthesis of crown ether derivatives. Its synthesis method is to use dibenzo-18-crown ether-6 as raw material and obtain it through nitration and reduction reactions. The reduction method used is Pd/C reduction method. Limited by expensive catalysts. The traditional synthesis method of dibenzo-18-crown ether-6, the raw material for the synthesis of diaminodibenzo-18-crown ether-6, is a reflux reaction under nitrogen protection. The conditions are harsh, the steps are cumbersome, the reaction cycle is long, and the temperature is high. Yield is low. Ultrasonic synthesis method is a new synthesis method developed in recent years. It has the advantages of good directionality, large energy, and strong penetrating ability. It is more convenient and easier to operate than traditional organic synthesis methods. The experimental equipment is also relatively simple and easy to control. It is increasingly used in organic synthesis.
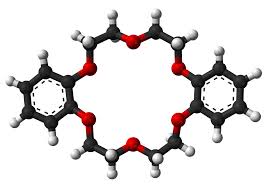
Preparation[1]
Preparation of dibenzo-18-crown ether-6: weigh a certain amount of catechol, bisdichloroethyl ether, KOH, a certain volume of DMSO and a small amount of 2,6-di-tert-butyl For p-cresol, seal it and put it into an ultrasonic reaction. Heat it to 50-60°C and keep it warm for 3 hours. Add water while it is hot and suction-filter out the black viscous substance. Wash with water and alkali-wash the filter cake. Let the filtrate stand and gradually precipitate an off-white solid. After suction filtration and recrystallization with methanol, 2.53g of product was obtained with a yield of 35.1%.
Main reference materials
[1] CN201210581030.7 Preparation method of diaminodibenzo-18-crown ether-6 bonded silica gel stationary phase and its intermediates

 微信扫一扫打赏
微信扫一扫打赏

