Background and overview[1]
2-Bromo-4′-cyanoacetophenone is a pharmaceutical intermediate that can be prepared from 4-cyanoacetophenone in one step.
Preparation[1]
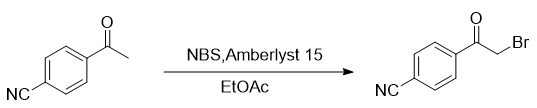
Add 10mmol 4-cyanoacetophenone and 11mmol N-bromosuccinimide (NBS) into a 100mL round bottom flask, dissolve 35mL ethyl acetate, then add 1g Amberlyst15 ion exchange resin as a catalyst, and mix the reaction solution The temperature was raised to 40°C for reaction. After the reaction was followed by TLC, the reaction solution was filtered to remove the Amberlyst15 ion exchange resin. The filtrate was spun dry and separated by column chromatography (eluent: petroleum ether/ethyl acetate) to obtain a white solid with a yield of 61%.
Apply[1]
CN201610570760.5 reported that 2-bromo-4′-cyanoacetophenone can be used to prepare the compound 2-(4-trifluoromethylphenylimino)-4-(4-cyanophenyl)thiazole, This compound is a new polymyxin B antibacterial synergist with high activity and excellent antibacterial synergistic activity. This type of compound can be used to prepare and treat a series of diseases related to Acinetobacter baumannii and Klebsiella pneumoniae, effectively reducing the dosage of polymyxin B and effectively reducing the toxicity of polymyxin B during the treatment process. risk, and can be applied to the antibacterial treatment of Acinetobacter baumannii and Klebsiella pneumoniae that are insensitive to polymyxin B or have weak antibacterial activity. The preparation method is as follows: add 1mmol N-(4-trifluoromethylphenyl) thiourea and 1.05mmol 2-bromo-4′-cyanoacetophenone into a 25mL eggplant-shaped bottle, add 10mL ethanol to dissolve, and then add 1.5mmoL triethyl Amine, reflux reaction. After the TLC tracking reaction, the temperature of the reaction solution was lowered to room temperature, the solvent was evaporated under reduced pressure, and the residue was separated by column chromatography (eluent: petroleum ether-ethyl acetate) to obtain the target compound as a yellow solid, with a yield of 63%. 1HNMR(400MHz, DMSO-d6)δ10.81(s,1H),8.14(d,J=8.3Hz,2H),7.94(d,J=8.6Hz,2H),7.91 (d,J=8.4Hz,2H),7.77(s,1H),7.72(d,J=8.6Hz,2H);19FNMR(376MHz,DMSO-d6)δ-59.91( s); HRMS(ESI)m/z[M+H]+ C17H11F3N3S+ Calculated value: 346.0626, measured value: 346.0631.
Main reference materials
[1][China Invention] CN201610570760.5 An antibacterial synergist and its preparation method and use

 微信扫一扫打赏
微信扫一扫打赏

