Overview[1]
1-(4-hydroxyphenyl)piperazine dicyanobromide can be used as a pharmaceutical synthesis intermediate, such as the preparation of 1-acetyl-4-(4-hydroxyphenyl)piperazine, ketoconazole (Ketoconazole) ) is a highly effective, low-toxic, orally effective broad-spectrum antifungal drug. One of its key intermediates is 1-acetyl-4-(4-hydroxyphenyl)piperazine.
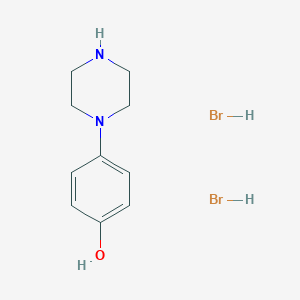
Preparation[1]
1-(4-hydroxyphenyl)piperazine dicyanobromide is prepared as follows:
1) Synthesis of dibromoethylamine hydrobromide
Add 194ml of diethanolamine (210g, 2.0mol) into a 2000ml three-necked flask, add 1050ml of 40% HBr dropwise under cooling and stirring (completed in about 2.5 hours), install the reaction bottle on a fractionating column (35cm high) and heat to 120 -130°C, distill off the generated water (collect 500ml in 40 hours). Raise the temperature to 140-150°C and evaporate excess hydrobromic acid (bp124°C) until the reaction solution becomes thick. After cooling slightly, add acetone and mix well. After cooling, crystals will precipitate. After filtering, wash with acetone and drain to obtain 319.7g (yield 51.2%), product mp166-170°C. The filtrate was concentrated under reduced pressure to precipitate crystals, and the product was recrystallized from acetone to obtain 27.5g of dibromoethylamine hydrobromide, with an mp of 142-148°C.
2) Synthesis of 4-methoxyphenylpiperazine dihydrobromide
In the reaction bottle, add 15.6g (0.05mol) of dibromoethylamine hydrobromide and a little methanol, then add 40ml of n-butanol and heat to dissolve, then add 6.19g (0.05mol) of p-aminoanisole. Stir and reflux at about 110°C for 6 hours. Add 5.2g of anhydrous sodium carbonate powder in portions while continuing to heat and stir. Heat and stir for 6 hours. After cooling slightly, pour the solution out and crystallize under ice-cooling. Filter the crystals with cold acetone. After washing, 7g of product 4-methoxyphenylpiperazine dihydrobromide was obtained, and its mp was 216-222°C. The product can also be obtained by refining the solid in the bottle with water to remove inorganic salts, totaling about 8g or more (yield 60-66%), product mp218-219°C.
3) Synthesis of 4-hydroxyphenylpiperazine dihydrobromide
Add 300ml of recovered 40% HBr and 39g (0.143mol) of 4-methoxyphenylpiperazine dihydrobromide into the reaction bottle, install it on a fractionating column (35cm high), heat to reflux, and slowly distill out about 88ml of water. Then increase the temperature to 124°C and continue distillation for 6 hours, then concentrate the reaction solution under reduced pressure to a thick consistency, add 50 ml of acetone after slightly cooling, shake, cool, filter the crystals and wash with acetone to obtain the product 4-hydroxyphenylpiperazine. Dihydrobromide 38g (yield 78.6%), its mp280°C (decomposition).
Main reference materials
[1] CN1616440

 微信扫一扫打赏
微信扫一扫打赏

