Background and overview[1]
5,6-Dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-1-piperidyl)phenyl]-2(1H)-pyridone is a One of the impurities in Pixaban. Apixaban is an oral, selective activated factor X inhibitor jointly developed by Pfizer and Bristol-Myers Squibb. In January 2013, it obtained the import drug license issued by the State Food and Drug Administration of China and was officially launched in China in April 2013. Apixaban is a new oral anticoagulant drug. Compared with other drugs, it can effectively prevent venous thromboembolism without increasing the risk of bleeding. It does not require routine monitoring of coagulation function or dose adjustment. It is mainly used clinically for prevention and Medicines to treat blood clots.
The main synthesis of apixaban is mainly from the compound SM-1(1-(4-aminophenyl)-5,6-dihydro-3-(4-morpholine)-2(1H)-pyridine Ketone) and compound SM-2 (5-chlorovaleryl chloride) are condensation cyclized to generate the key intermediate APSB-1 (5,6-dihydro-3-(4-morpholinyl)-1-[4-(2- Oxo-1-piperidinyl)phenyl]-2(1H)-pyridone), followed by reaction with SM-3 ([(4-methoxyphenyl)hydrazino]ethyl chloroacetate) to form the intermediate APSB-2(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7- Tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylic acid ethyl ester), and finally aminolysis with formamide to obtain apixaban. In the process of preparing the key intermediate APSB-1 from the reaction of SM-1 and SM-2, the dihydropyridone ring in the APSB-1 structure is easily oxidative and dehydrogenated to produce the pyridone impurity 5,6-dihydro-3-( 4-morpholinyl)-1-[4-(2-oxo-1-piperidinyl)phenyl]-2(1H)-pyridone. Drug impurities are very important in pharmaceutical process research, so the research on the above-mentioned apixaban and pyridone impurities is also very necessary.
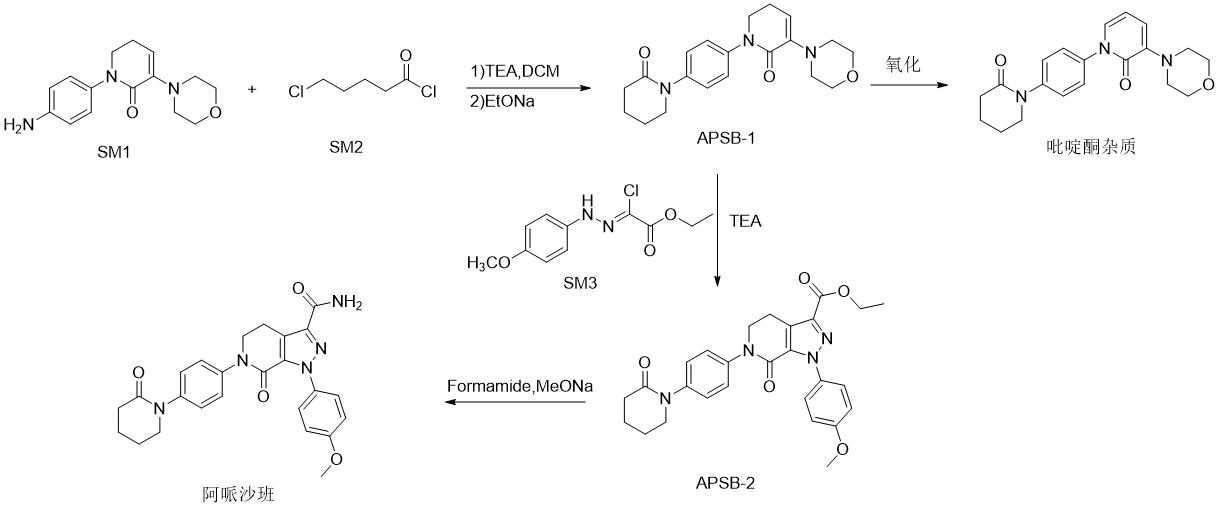
Preparation[1]
5,6-Dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-1-piperidyl)phenyl]-2(1H)-pyridone is prepared as follows :
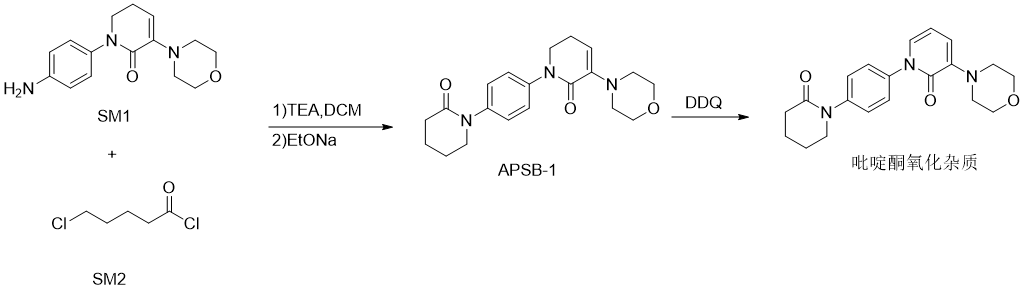
Add 150 mL dichloromethane, 1.6 g triethylamine and 3.5 g raw material SM-1 into a 250 mL four-neck reaction bottle. Add 2.4g of the raw material SM-2 dropwise in an ice bath. After the addition is completed, control the internal temperature to 10-30°C and keep stirring for 1 hour. Then, add 4.5g of sodium ethoxide solid into the reaction bottle. Raise the temperature to 10-30°C and keep stirring for 2 hours. After the reaction, add 100 mL of drinking water, stir for 30 minutes, pour into a separatory funnel and separate layers. Extract the upper aqueous liquid once with 100 mL of methylene chloride, combine the lower organic phases and concentrate to obtain 5.2 g of APSB-1 solid. Add 5.2g of the above APSB-1 solid to the 250mL reaction bottle, add 6.67g of 2,3-dichloro-5,6-dicyanobenzoquinone, add 150mL of dichloromethane, replace with nitrogen three times, raise the temperature to 50°C and reflux for 24 hours, after the reaction is completed, it is cooled to room temperature, 150 mL of saturated sodium carbonate solution is added, the water layer is extracted once with 150 mL of dichloromethane, the organic layers are combined, dried over anhydrous sodium sulfate, concentrated to obtain a solid, and column chromatography (petroleum ether: acetic acid Ethyl ester=3:1-1:5) was separated and purified to obtain light yellow solid pyridone impurity 5,6-dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-1- Piperidinyl)phenyl]-2(1H)-pyridone 3.1g. HPLC: 99.02%; ESI-MSm/z=353.9([M+H]+), 376.0([M+Na]+), 707.2([2M+ H]+), 729.1([2M+Na]+); 1H-NMR(CDCl3 , 400MHz): δppm: 7.38-7.44(m, 4H), 7.15-7.24(m, 2H), 6.37-6.41(m, 1H), 3.92-3.98(m, 4H), 3.70-3.73(t, 2H) , 3.35-3.36 (m, 4H), 2.66-2.69 (m, 2H), 2.00-2.01 (m, 4H).
Main reference materials
[1] CN201910733938.7 Preparation method of apixaban pyridone impurity

 微信扫一扫打赏
微信扫一扫打赏

