Background and overview[1]
2-Bromo-5-methoxyphenylboronic acid can be used as a pharmaceutical synthesis intermediate and can be prepared from 3-methoxyphenylboronic acid, triphenylphosphine sulfide and N-bromosuccinimide.
Preparation[1]
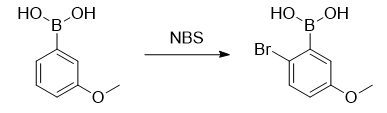
Under an argon atmosphere, add 3-methoxyphenylboronic acid (9.12g, 60.0mmol), triphenylphosphine sulfide (932mg, 6.00mmol), and dichloromethane (200mL) into a 300 ml eggplant-shaped flask. ), stir it to form a homogeneous solution. N-bromosuccinimide (13.0 g, 72.0 mmol) was added thereto, and the mixture was stirred at room temperature for 2 days. A saturated sodium thiosulfate aqueous solution was added to the reaction mixture to terminate the reaction, and then extracted with dichloromethane (100 mL × 3). The combined organic layers were washed with saturated brine and then dried over sodium sulfate. After filtration, it was concentrated under reduced pressure to obtain crude product. It was purified by silica gel column chromatography (290g, n-hexane/acetone = 1/1) to obtain 2-bromo-5-methoxyphenylboronic acid (8.45g, 36.6mmol), yield 61.0%).
Apply[1]
2-Bromo-5-methoxyphenylboronic acid can be used to prepare 2-bromo-5-methoxyphenyl)methyl sulfide: Under an argon atmosphere, anhydrous copper (II) sulfate (79.8 mg, 0.500 mmol) and sodium bicarbonate (840 mg, 10.0 mmol) were added to a 100 mL eggplant-shaped flask. On the other hand, p-tolylmethylthiosulfonate (1.01 g, 5.00 mmol) and 2-bromo-5-methoxyphenylboronic acid (1.73 g, 7.50 mmol) were dissolved in methanol (30 mL). The above solution was added and the mixture was stirred at room temperature for 4 days. Water was added to the reaction mixture to terminate the reaction, and the mixture was extracted with ethyl acetate (50 mL × 3). The combined organic layers were washed with saturated brine and then dried over sodium sulfate. After filtration, it was concentrated under reduced pressure to obtain crude product. Purified by silica gel column chromatography (30g, n-hexane/dichloromethane=5/1), (2-bromo-5-methoxyphenyl)methyl sulfide (835mg, 3.62mmol) was obtained, with a yield of 72.4 %.
Main reference materials
[1] JP2018030788 METHOD FOR PRODUCING PHENOTHIAZINE DERIVATIVE

 微信扫一扫打赏
微信扫一扫打赏

