Background and overview[1-2]
2-Bromo-1-chloro-4-trifluoromethoxybenzene is a pharmaceutical intermediate, which can be prepared from 2-bromo-4-(trifluoromethoxy)aniline after diazotization and chlorination. 2-Bromo-1-chloro-4-trifluoromethoxybenzene can be used to prepare triazolecarboxylic acid derivatives (such as 4-((4′-chloro-3′-(trifluoromethoxy)-[1, 1,-Biphenyl]-4-yl)thio)-1H-1,1,2,3-triazole-5-carboxylic acid), these derivatives can be used as glycolate oxidase inhibitors.
Preparation[1-2]
Report 1,
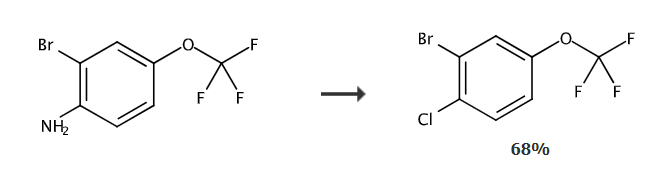
A solution of sodium nitrite (0.81g, 11.72mmol) in water (2mL) was added dropwise to 2-bromo-4-(trifluoromethoxy)aniline (1.18mL, 7.81mmol) at 5°C. in 6N hydrochloric acid solution. The mixture was stirred at this temperature for 40 minutes and then a solution of CuCl (1.55 g, 15.62 mmol) in concentrated HCl (3 mL) was added dropwise. The mixture was allowed to warm to room temperature and stirred for 2-6 hours. DCM and water were added and the product was extracted twice. The combined organic layers were dried, filtered and concentrated. The crude compound was purified by flash column chromatography (20 g silica gel, heptane:AcOEt from 100:0 to 85:15) to give 2-bromo-1-chloro-4-trifluoromethoxybenzene (1.47 g, 68%).
Report 2,
Combine isopentyl nitrite (4 mL, 30 mmol), copper(II) chloride (3.22 g, 24 mmol) and 4-bromo-2-(trifluoromethoxy)aniline (5.1 g, 20 mmol) in acetonitrile (80 mL) was heated at 70°C for 3 hours. The mixture was poured into aqueous HCl solution (0.5M, 50mL) and extracted with ethyl acetate (50mL×2). The combined extracts were washed with water (50 mL × 4) and brine (50 mL), dried over anhydrous sodium sulfate, filtered and concentrated. The residue was purified by silica gel flash column chromatography (petroleum ether) to obtain compound 2-bromo-1-chloro-4-trifluoromethoxybenzene.
Main reference materials
[1] Rombouts F J R , Tresadern G , Buijnsters P , et al. Pyrido[4,3- e ][1,2,4]triazolo[4,3- a ]pyrazines as Selective, Brain Penetrant Phosphodiesterase 2 ( PDE2) Inhibitors[J]. Acs Medicinal Chemistry Letters, 2015, 6(3):150115135115001.
[2]From PCT Int. Appl., 2019133770, 04 Jul 2019

 微信扫一扫打赏
微信扫一扫打赏

