Background and overview[1]
2,4-Diphenyl-oxazacyclo-5-acid is a pharmaceutical intermediate that can be prepared in two steps from (2R,3S)-3-phenylisoserine and trimethyl orthobenzoate. get. It has been reported in the literature that 2,4-diphenyl-oxazacyclo-5-acid can be used to introduce substituents at the C-13 and C-14 positions of paclitaxel, retaining the ring skeleton and necessary functional groups of taxanes, and the resulting compound It has the anti-cancer biological activity of natural paclitaxel and has certain application prospects in reducing the toxic and side effects of natural paclitaxel.
Preparation[1]
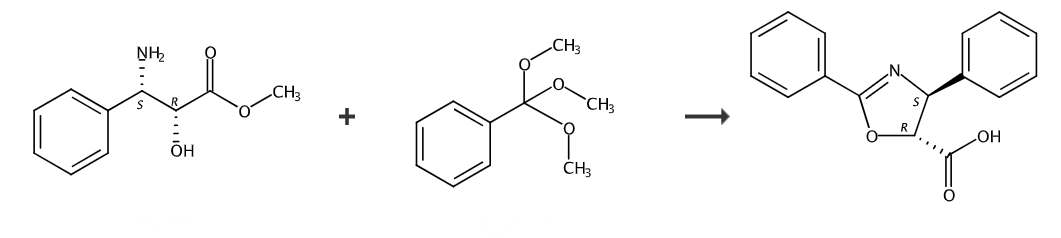
Dissolve (2R,3S)-3-phenylisoserine (2.87g, 0.013mol) in 50ml of dry toluene, increase the temperature to 110°C, and then add trimethyl orthobenzoate (5ml, 0.029 mol), after 8 hours of reaction, the raw material disappeared completely according to TLC. The solvent was evaporated under reduced pressure, and the initial product was slowly separated by column chromatography (thin layer chromatography on silica gel, petroleum ether: ethyl acetate = 8:1) to obtain a white product (4S, 5R)-2,4-diphenyloxane. Azole-5-carboxylic acid methyl ester, yield 66%. 1H NMR (200MHz): δH 3.90 (3H s), 4.96 (1H, d, J = 6.5Hz,), 5.50 (1H, d, J = 6.5Hz,), 7.30-7.62 (8H,m),8.10-8.20(2H,m);13C NMR (CDCl3,50MHz): δC 52.68,74.59,83.07,126.39,126.72, 127.97,128.15,128.22,128.39,128.64,128.79,131.86,141.04,163.89,170.55.
Dissolve (4S,5R)-2,4-diphenyloxazole-5-carboxylic acid methyl ester (1.52g, 0.0054mol) in 8ml of methanol, and then add sodium hydroxide (0.33g, 0.00835mol ) was dissolved in a mixed solution of 20 ml methanol and 0.5 ml water, stirred at room temperature, and reacted for about 5 hours. Track the reaction with TLC and observe the formation of new points. Then use 5% hydrochloric acid solution to quench the reaction and adjust the pH to 6. The low-boiling solvent was evaporated under reduced pressure and dissolved in ethyl acetate. The organic phase was washed with water several times and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure to obtain 2,4-diphenyl-oxazacyclo-5 as a white solid. -Acid, yield 86%. 1H NMR (200MHz, DMSO-d6,): δH 5.00 (1H, d, J = 6.4Hz,), 5.41 (1H d, J = 6.4Hz,), 7.20-7.70 (8H ,m),7.90-8.10(2H,m);13C NMR(DMSO-d6,500MHz)δC73.78,82.42,126.53,126.64,127.79,128.18,128.71,128.80,132.09, 141.54,162.86,171.45.
Main reference materials
[1] [China invention, China invention authorization] CN201710250423.2 Paclitaxel compounds with structural modification at C-13 and C-14 positions and their preparation method [Authorization]

 微信扫一扫打赏
微信扫一扫打赏

