Background and overview[1]
2-(2-Methoxy-4-niphenyl)-3-(4-niphenyl)-5-(2,4-disulfobenzene)-2H-tetrazole monosodium salt, WST-8, referred to as WST-8, is a main component in the cell activity detection agent CCK-8 kit. CCK-8 kit is a kit developed by Japan’s Dojindo Research Institute for detecting cell proliferation and cytotoxicity. The kit is easy to use, has high sensitivity, good repeatability and is non-radioactive. However, the kit is expensive, and its main component, WST-8, is sold at a high commercial price.
Preparation[1]
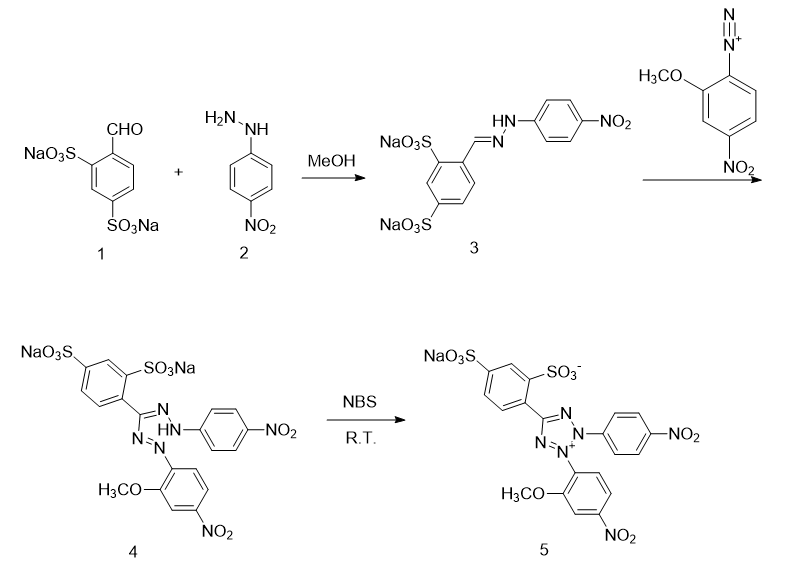
1) Preparation of compound 3
Put compound 2 (4.94g, 0.0322mol) into a 500ml three-necked flask, add 150ml methanol, heat and stir until reflux dissolves. Cool to 50°C and slowly add compound 1 (10.0 g, 0.0322 mol). Keep the temperature at 50°C and continue stirring for 2 hours. Filtration under reduced pressure and washing with a small amount of methanol gave compound 3 (11.065g, 0.0249mol) with a yield of 77.4%.
1H-NMR (400MHz, D2O) δH: 6.83 (d, 2H, J=9Hz), 7.65 (dd, 1H, J=8Hz , 1Hz), 7.81 (d, 2H, J=9Hz), 7.93 (d, 1H, J=8Hz), 8.03 (d, 1H, J=1Hz), 8.31 (s, 1H).
2) Preparation of 4-nitro-2-methoxydiazonium salt
Put 2-methoxy-4-nitroaniline (3.7g, 0.0220mol) in a 50ml single-mouth bottle, place it in an ice bath at -10~0°C, and add 15ml of 6.67ml of concentrated hydrochloric acid diluted with water. Stir for 5 minutes. Then slowly add dropwise (1.64g, 0.0237mol) a solution of sodium nitrite dissolved in 10ml of water. After the addition is complete, continue stirring for 30 minutes. That is, diazonium salt solution is obtained. Reserve (ready to use).
3) Preparation of compound 4
Put compound 3 (10.0g, 0.0224mol) into a 500ml three-necked flask, add 200ml of methanol, and stir for 5 minutes. Then, the above diazonium salt solution was added while adding 15 ml of NaOH (3.6 g, 0.09 mol) aqueous solution. After the addition is completed, stir in an ice bath at -10~5°C for 2 hours and at room temperature for 2 hours. At this time, the reaction system turns dark green. Use 4 ml of concentrated hydrochloric acid and 8 ml of hydrochloric acid solution diluted with water to make it dark red. Evaporate the solvent to dryness. Add 200 ml of petroleum ether and perform ultrasonic filtration under reduced pressure to obtain compound 4 (12.054 g, 0.0193 mol) as a reddish-brown solid with a yield of 86.2%.
1H-NMR (400MHz, D2O) δH: 3.65 (s, 3H), 7.42 (t, 4H), 7.50 (d, 1H, J=2Hz), 7.69 (d, 1H, J=9Hz), 8.03 (m, 3H), 8.33 (d, 1H, J=1Hz).
4) 2-(2-methoxy-4-nitrobenzene)-3-(4-nitrobenzene)-5-(2,4-disulfobenzene)-2H-tetrazole mono Preparation of sodium salt
Put compound 4 (12.054g, 0.0193mol) into a 500ml single-neck bottle, add 120ml of methanol, and it will turn dark reddish brown. Stir for 5 minutes. Add 100 ml of acetone solution of NBS (5.0 g, 0.0281 mol). Stir for about 1 hour, the color becomes lighter, continue stirring until the reaction system turns yellow. Filter under reduced pressure to obtain a yellow-green solid. Recrystallize (ethanol/water = 2) and dry in vacuum to obtain compound 2-(2-methoxy-4-nitrobenzene)-3-(4-nitrobenzene)-5-(2,4-disulfonate) Benzene)-2H-tetrazole monosodium salt (6.211g, 0.0099mol), light yellow solid powder, yield 51.6%.
1H-NMR (400MHz, D2O) δH: 3.54 (s, 3H), 7.95 (m, 6H), 8.13 (d, 1H, J=8Hz), 8.34 (m, 3H); 1H-NMR (400MHz, d6-DMSO) δH: 3.74 (s, 3H), 7.86 (m, 2H), 8.06 (d, 1H, J=2Hz), 8.14 (d, 2H, J=9Hz), 8.24 (dd, 1H, J=8Hz, 2Hz), 8.30 (d, 1H, J=1Hz), 8.54 (d, 3H, J=9Hz).
Main reference materials
[1]CN201210358733.3 2-(2-methoxy-4-niphenyl)-3-(4-niphenyl)-5-(2,4-disulfobenzene)-2H- Synthesis method of tetrazole monosodium salt

 微信扫一扫打赏
微信扫一扫打赏

