Background and overview[1]
6,7-Dihydro-4(5H)-benzofuranone is an important pharmaceutical intermediate. It can be prepared by the reaction of 1,3-cyclohexanedione and chloroacetaldehyde aqueous solution, and can be used to synthesize 5 -Acetyl-6,7-dihydro-4(5H)-benzofuranone.
Preparation[1]
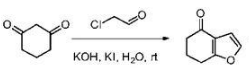
Synthesis of 6,7-dihydro-4(5H)-benzofuranone: Add 1,3-cyclohexanedione (10.0g, 89.18mmol) to a 250mL round-bottomed flask, and dissolve it in 60mL of water. Add 35 mL of 20% sodium hydroxide solution under ice bath, then add KI (2.9g, 17.4mmol), and finally slowly add 16 mL of 40% chloroacetaldehyde aqueous solution dropwise, complete the addition, and react at room temperature. After 12 hours, the TLC test shows that the raw materials are basically the same. Complete response. Add 37.5% concentrated hydrochloric acid to adjust to pH=4, add 100mL of water, extract with petroleum ether (100mL×4), combine the organic phases, wash with saturated brine (100mL×3), and finally use anhydrous sodium sulfate. Dry and evaporate the solvent under reduced pressure to obtain 10.0g of light yellow liquid 7, namely 6,7-dihydro-4(5H)-benzofuranone, with a yield of 82%. 1HNMR (400MHz, CDCl3) δ: 7.32 (d, J=2.0Hz, 1H), 6.67 (d, J=2.0Hz, 1H), 2.90 ( m, 2H), 2.52 (m, 2H), 2.21 (m, 2H).
Apply[1]
Synthesis of 5-acetyl-6,7-dihydro-4(5H)-benzofuranone: Add 6,7-dihydro-4(5H)-benzofuranone to a 250mL round bottom flask (7.0g, 51.45mmol), dissolve it in 30mL anhydrous tetrahydrofuran, add 60% NaH (8.23g, 205.8mmol) in an ice bath, complete the addition, fill with nitrogen for protection, add ethyl acetate dropwise after 1 hour, complete the addition, and react at room temperature , after 2 hours, TLC detection showed that the raw materials were basically completely reacted. Add 1mol·L-1HCl to adjust the pH to about 7, add 100mL water, extract with ethyl acetate (100mL×3), combine the organic phases, wash with saturated brine (100mL×3), and finally dry with anhydrous sodium sulfate. The solvent was evaporated to dryness under reduced pressure to obtain 9.0 g of yellow oily substance 8, namely 5-acetyl-6,7-dihydro-4(5H)-benzofuranone, with a yield of 98%. 1HNMR (400MHz, CDCl3) δ: 7.34 (d, J=2.0Hz, 1H), 6.67 (d, J=2.0Hz, 1H), 3.61 ( t, J=4.0Hz, 1H), 3.19 (m, 1H), 2.91 (m, 1H), 2.60 (m, 1H), 2.31 (s, 3H), 2.28 (m, 1H).
Main reference materials
[1]CN201910456743.2 Preparation method and application of aquabacterin

 微信扫一扫打赏
微信扫一扫打赏

