Background and overview[1-2]
IR-813 p-toluenesulfonate is a cyanine dye and an infrared absorber. There are reports in the literature that IR-813 p-toluene sulfonate can be used to prepare a positive-working infrared sensitive composition with good resistance to solvents and printing room chemicals.
Preparation[1]
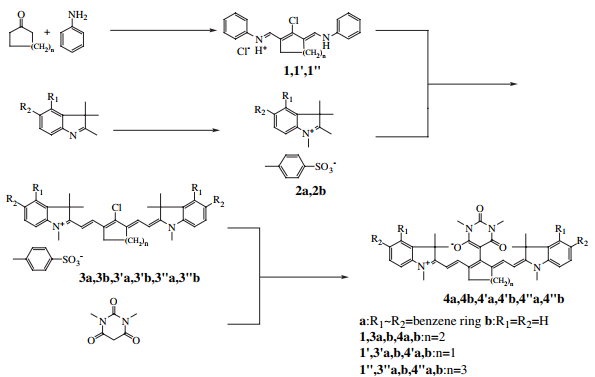
IR-813 p-toluenesulfonate is compound 3a in the picture above. The preparation method is as follows:
At 80°C, a solution of indole salt 2a (6mmol, 2.37g), aniline salt 1 (3mmol, 1.179g) and sodium acetate (7mmol, 600mg) in ethanol (60ml) was heated at 80°C under nitrogen atmosphere. Stir for 1 hour. After removing the solvent, the product was purified by silica gel column chromatography (eluent solvent; chloroform:methanol=19:1) to obtain IR-813 p-toluenesulfonate (0.600g, 81.6%). 1HNMR(300MHz,DMSO-d):7.47e8.39(m,16H),7.11(d,J=7.8Hz,2H,AreH),6.35(d,J=14.4Hz, 2H,eCH]),3.82(s,6H,eCH3),2.76(m,4H,eCH2e),2.28(s,3H,eCH3),1.96(s,12H,eCH3),1.91(m,2H,eCH2e) .
Apply[2]
IR-813 p-toluenesulfonate is used to prepare positive-image infrared sensitive compositions. The method is as follows:
Positive infrared sensitive compositions include:
IR-813 p-toluenesulfonate, 0.2 parts by weight, purchased from DKSH Holding Ltd.;
Methyl violet, 0.14 parts by weight;
Diphenyliodonium hexafluorophosphate, 0.20 parts by weight;
Polymer, 6.16 parts by weight; the polymer includes a main chain LB6564 resin with phenolic repeating units and a substituent group covalently connected to the main chain LB6564 resin. The preparation method of the polymer is as follows: In a closed fume hood, add 5 parts by weight of dry LB6564 resin (purchased from Becklight, Germany) and 40.8 parts by weight of DMF into a four-necked round-bottomed flask equipped with mechanical stirring, a thermometer, a reflux condenser and a constant pressure funnel. , stir until LB6564 resin is completely dissolved. After the DMF solution of the LB6564 resin is clarified, 0.5 parts by weight of melamine, 1.1 parts by weight of pyromellitic dianhydride and 0.5 parts by weight of hexamethylenetetramine are added in sequence, and the temperature is raised to 154°C to allow the reaction solution to react in a reflux state. 8h. After the reaction is complete, filter, and drop the filtrate into 750 parts by volume of water while stirring. After the dropwise addition is completed, add a mixture of 1.25 parts by volume of hydrochloric acid and 23.75 parts by volume of water to the above aqueous solution, and stir for 2 hours. The precipitate was separated out, and after suction filtration, washing with distilled water and drying, the polymer (modified LB6564 resin) was obtained. The molecular weight of the modified LB6564 resin was determined by GPC to be 8,000.
Dissolve 6.7 parts by weight of the positive infrared sensitive composition prepared above in a mixed solvent of 46.65 parts by weight of PM and 46.65 parts by weight of DMF, and apply the solution on the electrochemically roughened and anodized surface using a rotating method. The treated aluminum plate base was then dried in an oven at 140°C for 2 minutes to prepare a lithographic printing plate precursor with a coating weight of 1.5g/m2.
The positive-image infrared-sensitive composition prepared by the above method is relatively sensitive to radiation with a maximum wavelength of 700 to 1300 nm. It is an excellent infrared-sensitive composition, and the lithographic printing plate precursor made from the composition It also has good tolerance to the erosion of isopropyl alcohol. Therefore, the imageable layer prepared by using the positive-image infrared-sensitive composition described in this method is not prone to erosion and dissolution of printing chemicals during use, thus Extend the life of lithographic printing plate precursors.
References
[1]NagaoY,SakaiT,KozawaK,etal.Synthesisandpropertiesofbarbiturateindolenineheptamethinecyaninedyes[J].Dyes&Pigments,2007,73(3):344-352.
[2][China invention, China invention authorization] CN201410222788.0 positive image infrared sensitive composition and imageable component thereof

 微信扫一扫打赏
微信扫一扫打赏

