Background and overview[1-2]
Haloformic acid and its derivatives are an important class of chemical and pharmaceutical intermediates, which are widely used as raw materials for the production of fungicides, pesticides, dyes and drugs. 3,5-Dichlorobenzoic acid is one of them. kind. 2,3-Dichlorobenzoic acid is a synthetic anti-epileptic drug Lamotrigine (3,5-diamino-6-(2,3-dichlorophenyl)-1,2,4-triazine) And other drugs for the treatment of central nervous system disorders such as key intermediates of 2,4-diamino-6-(2,3-dichlorophenyl)pyrimidine.
Preparation[1-2]
Report 1,
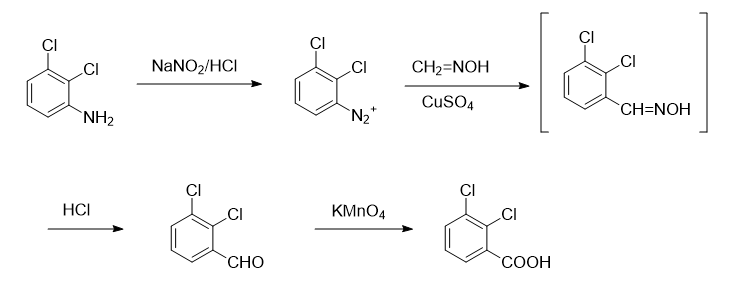
Synthesis of 1, 2,3-dichlorobenzaldehyde
Add 16.2g (0.1mol) of 2,3-dichloroaniline, 60mL of water and 30mL of concentrated hydrochloric acid into a 500mL three-neck flask to generate a white solid. Heat and reflux with stirring until the solid is completely dissolved to obtain an orange transparent solution. Use Cool the ice-salt bath to 0~5℃, during which a pink precipitate will precipitate. Add dropwise a solution composed of 7g sodium nitrite and 10mL water, control the temperature at 0~5℃, complete the dropwise addition, continue stirring for 15min, and add 9.8g A solution of hydrated sodium acetate dissolved in 16 mL of water until the solution is neutral to Congo red test paper. The diazonium salt solution is cooled until use. In addition, add 4.6g paraformaldehyde, 10.5g hydroxylamine hydrochloride and 68mL water into a 1000mL three-neck flask, stir and heat until the solid is completely dissolved (this process takes about 20min), then add 20.4g hydrated sodium acetate, heat and reflux for 15min to prepare Obtain w (formaldehyde oxime) = 10% aqueous solution. Add 2.6g copper sulfate hydrate, 0.4g sodium bisulfite, 32g hydrated sodium acetate and 12.5g sodium carbonate in 72mL water to the formaldehyde oxime solution, use an ice bath to maintain the temperature at 10-15°C, and stir quickly. Add diazonium salt solution dropwise. Continue stirring for 1 hour, add 92 mL of concentrated hydrochloric acid, heat to reflux for 2 hours, and then perform steam distillation until no 2,3-dichlorobenzaldehyde evaporates. Collect the distillate (about 800 mL). After cooling, filter to obtain light yellow crude product. Add 36 mL w (sodium bisulfite) = 40% aqueous solution to a 100 mL three-neck flask equipped with a condenser tube, dropping funnel and thermometer, stir magnetically, heat the water bath to 60°C, and remove the crude 2,3-dichlorobenzaldehyde. Dissolve an appropriate amount of tetrahydrofuran and add it dropwise to the system. Keep the reaction temperature at 60°C. During this period, a white solid will be generated. After the dropwise addition is completed, after stirring for 0.5h, turn off the condensed water, continue stirring for 1h, and let it stand overnight. A large amount of white solid will precipitate. . Filter with suction, wash the solid with anhydrous ether (30mL Dichlorobenzaldehyde 7.9g, yield 48%, m.p.: 66.4~67.2℃, content more than 99% (gas chromatography). The literature yield is 32.6%, m.p.: 64~67℃.
Synthesis of 2, 2,3-dichlorobenzoic acid
Add 8.7g (0.05mol) of 2,3-dichlorobenzaldehyde and 180mL of water into a 500mL three-necked flask equipped with mechanical stirring and dropping funnel, heat to 70~80℃, stir for 20min Add dropwise a solution of 11.3g potassium permanganate in 225mL water. After the dripping is complete, heat and stir for 1 hour. Add w(KOH)=10% aqueous solution to make the reaction solution pH=11~12. Filter and wash with hot water. 3 times, combine the filtrate and washing liquid, cool, filter, acidify the filtrate with hydrochloric acid until no solid precipitates, filter with suction to obtain a crude product, dissolve the obtained crude product in about 150mL of aqueous solution w (NaOH) = 5%, add 1g Boil activated carbon for 10 minutes, filter while hot, acidify the filtrate with concentrated hydrochloric acid to pH=2, and precipitate more white crystals. After cooling, filter and dry to obtain 9.0g of white product 2,3-dichlorobenzoic acid, with a yield of 94 %, m.p.: 167.9~168.8℃ (literature value [1]: 167~169℃), purity 99.6% (gas chromatography), two-step total yield 45%. IR (KBr tableting), νmax/cm-1: 3002 (broad peak, carboxyl association νOH), 1694 (carboxylic acid νC=O); MS, m/Z: 190.8 (M+), 188.8, 189.8, 192.8, 144.8,145.8,146.8,148.8.
Report 2,
To 100.0g benzonitrile (content 99%), add 300g chloroform and 100g ethanol respectively, then add 1209.4g sodium hypochlorite (content 13%), and use 37% hydrochloric acid to maintain the pH value of the system at 3.0-4.0. At 55-60℃, react until the controlled content of 3,5-dichlorobenzonitrile is 99.5%, then add 30% sodium hydroxide under normal pressure to keep the pH value of the reaction system at 12-13, 80-90 After 2.5 hours of heat preservation reaction, use 37% hydrochloric acid to acidify the system until the pH value is 1.0. React for 1.0 hours at 50-60°C, then filter, and dry at 90-110°C to obtain 182.5g with a content of 99.0%. of 3,5-dichlorobenzoic acid.
Apply[3]
CN201410495742.6 discloses a flame-retardant epoxy resin composite material and a preparation method thereof. The composite material includes 100 to 200 parts by weight of epichlorohydrin, 200 to 220 parts of diphenolmethane, 50 to 90 parts of potassium carbonate, 100 to 180 parts of 5-chloro-N-methylisatoic anhydride, 2 , 40~100 parts of 3-dichlorobenzoic acid, 60~200 parts of N-methyl-4-aminophenol sulfate, 20~40 parts of p-methoxyphenylethylamine, 150~200 parts of 4-aminobutyric acid, 100~280 parts of organophosphorus flame retardant, 40~120 parts of polymetaphosphoric acid, and 40~80 parts of plasticizer. The preparation method is: weigh epichlorohydrin, diphenolmethane, 5-chloro-N-methylisatoic anhydride, and 2,3-dichlorobenzoic acid into a container and mix thoroughly, then add the remaining components and continue. Mix evenly, then transfer to open mill for hot mixing, flaking, cooling and crushing. The bending strength of the invention is greater than 200MPa, the heat deformation temperature is greater than 280°C, the insulation resistance is greater than 1.7×1014Ω, the flame retardancy reaches V-0 level, and it has excellent flame retardant properties.
References
[1] Liao Qi, Deng Hong. Research on the synthesis of 2,3-dichlorobenzoic acid[J]. Fine Chemical Intermediates, 2006(04):18-20.
[2][China invention, China invention authorization] CN201310184468.6 A method of synthesizing 3,5-dichlorobenzoic acid
[3]CN201410495742.6 A kind of flame retardantOxygen resin composite material and preparation method thereof

 微信扫一扫打赏
微信扫一扫打赏

