Background and overview[1]
2,6-Dichloro-4-trifluorotoluidine is a key intermediate of the pesticide fipronil. The chemical name of fipronil is 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethylsulfinylpyrazole, trade name Fipronite is a highly effective insecticide developed by the French Rhône-Poulenc company from 1987 to 1989 and registered in my country in 1992.
Preparation[1-2]
Report 1,
Preparation of 3-chloro-4-amino-5-nitrotrifluorotoluene
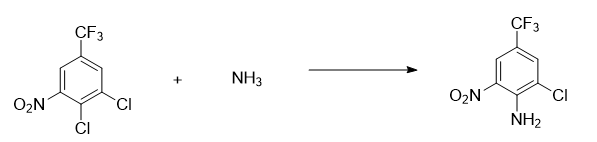
Add 600Kg of 3,4-dichloro-5-nitrotrifluorotoluene into the reaction kettle, seal the reaction kettle, replace it with nitrogen, start stirring, gradually raise the temperature to 160°C, and gradually introduce ammonia gas to the amount of When it reaches 125Kg, take a sample of 3,4-dichloro-5-nitrotrifluorotoluene and detect 0.5% through the sampling tube. Cool the temperature below 80℃, vent the ammonia gas to the tail gas recovery device, add 200Kg*2 water to the reaction kettle for washing, and organic The layer was washed with saturated brine and dehydrated under reduced pressure to obtain 544Kg of material (content 99.2%, moisture 0.5%, yield 98.0%).
Preparation of 2,6-dichloro-4-trifluoromethylaniline
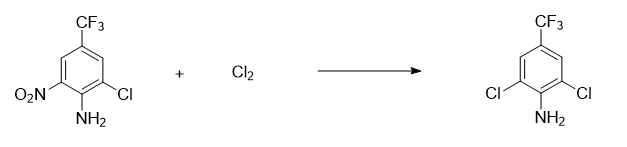
Add 1200Kg of 3-chloro-4-amino-5-nitrotrifluorotoluene into the chlorination kettle, seal the reaction kettle, replace it with nitrogen, start stirring, gradually raise the temperature to 50°C, gradually introduce chlorine gas, and control the reaction temperature 70-80℃, when the input volume reaches 407Kg, 3-chloro-4-amino-5-nitrotrifluorotoluene 0.5% is passed through the sampling tube, cool down to below 30℃, and vent the chlorine to the tail gas recovery device , add 200Kg of ammonia water to the reaction kettle*4, wash with water, dehydrate under reduced pressure, and distill under reduced pressure to obtain 1090Kg of material (content 99.0%, moisture 0.5%, yield 95.0%).
Report 2,
Put 200g of 3,4,5-trichlorotrifluorotoluene and 145g of water into the 1000ml high-pressure reactor, seal the reactor, and fill it with 380g of liquid ammonia. After adding, close the valve, start stirring, and slowly increase the temperature rise to 160°C, the pressure rises to 10Mpa, and timed heat preservation is performed. During the heat preservation period, the reaction temperature is controlled to 165°C, the pressure is 11.5Mpa, and the heat preservation time is 8 hours. When the heat preservation is completed, the pressure is released and ammonia is discharged.
After washing the reaction materials with water, the crude reaction product is distilled, and 20.4g of the unreacted raw material 3,4,5-trichlorotrifluorotoluene is recovered to obtain 120g of 2,6-dichloro-4-trifluoromethylaniline, content 99.24%, yield 72.48%.
Apply[3]
5-Amino-1-(2,6-dichloro-4-trifluoromethylphenyl)-3-cyanopyrazole is a highly effective broad-spectrum insecticide fipronil (its trade name is ” Fipronil”)’s key intermediate. CN200710156221.8 discloses a preparation method of 5-amino-1-(2,6-dichloro-4-trifluoromethylphenyl)-3-cyanopyrazole, using 2,6-dichloro-4 -Trifluoromethylaniline is used as raw material, 40~98% sulfuric acid is used as the reaction medium, an aqueous solution of sodium nitrite is added dropwise and the diazotization reaction is carried out at 0~70°C for 0.5~1 hour to obtain the diazotization reaction liquid. Adjust the temperature of the reaction solution to 15~30°C, add ethyl 2,3-dicyanopropionate, and then perform the cyclization reaction in an organic solvent under weakly alkaline conditions at 25~50°C. After the reaction is completed, post-processing The compound 5-amino-1-(2,6-dichloro4-trifluoromethylphenyl)-3-cyanopyrazole was obtained. The invention effectively reduces production costs and reduces the emission of “three wastes”, thereby maintaining the ease of reaction, improving the yield of the reaction, and reducing the emission of “three wastes”.
References
[1][Chinese invention, Chinese invention authorization] CN201710605773.6 A method for preparing 99% content of 2,6?dichloro?4?trifluoromethylaniline [Public]/A method of preparing 99% content of 2 , method of 6-dichloro-4-trifluoromethylaniline [Authorized]
[2][China invention, China invention authorization] CN200810060866.6 A method for preparing 2,6-dichloro-4-trifluoromethylaniline
[3][China invention, China invention authorization] CN200710156221.8 A kind of 5-amino-1-(2,6-dichloro-4-trifluoromethylphenyl)-3-cyanopyrazole Preparation method

 微信扫一扫打赏
微信扫一扫打赏

