Background and overview[1-2]
5-Methanoyl-2-methyl-benzonitrile is an organic intermediate. According to literature reports, it can be synthesized from 3-(dimethylammonium)acrylonitrile and (E,E)-2,4-hexane. Dienal is prepared in one step or in three steps from 4-methyl-3-nitrobenzyl alcohol.
Preparation[1-2]
Report 1,
5-Methanoyl-2-methyl-benzonitrile is 3t in the figure below, with a yield of 54%.
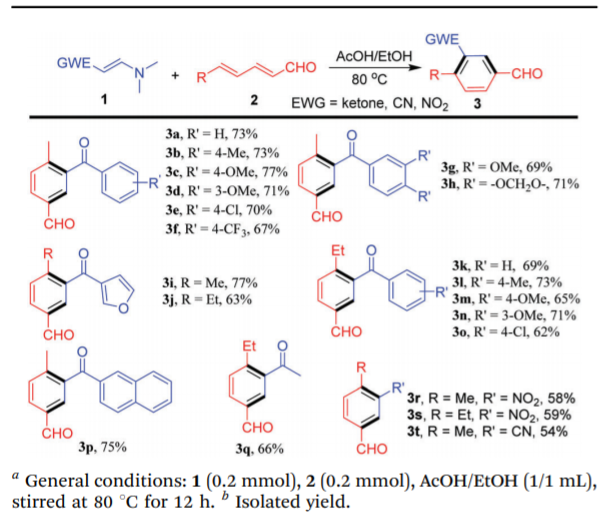
Dissolve 3-(dimethylammonium)acrylonitrile (0.2mol) and (E,E)-2,4-hexadienal (0.2mol) in acetic acid/ethanol (1/1ml), Stir at 80°C for 12 hours.
Report 2,
Step 1, preparation of 3-amino-4-methylbenzyl alcohol
4-Methyl-3-nitrobenzyl alcohol (9.7 g, 58 mmol) for 4 hours to obtain 7.54 g (93%) of the title compound after standard work-up. MS (DCI+) m/z: 138 (M+H)+.
Step 2, 3-cyano-4-methylbenzyl alcohol
To 3-amino-4-methylbenzyl alcohol (7.54g, 55mmol) in 2N hydrochloric acid (200mL) was added dropwise a solution of sodium nitrite (5.67g, 82mmol) in water (65mL) , keep the temperature below 10°C. After stirring for 2 hours, the diazonium salt solution was added to a suspension of copper cyanide (9.4 g, 0.1 mol) and sodium cyanide (3.0 g, 61 mmol) in water (75 mL), keeping the temperature below 10°C. The reaction mixture was allowed to warm to room temperature, stirred overnight, filtered through celite and extracted with ethyl acetate (3x). The organic extract was washed with 1N hydrochloric acid, brine, 5% sodium bicarbonate, water, dried (MgSO4), filtered and the solvent was evaporated. The crude residue was purified by flash chromatography on silica gel, eluting with ethyl acetate:hexane (3:7), to give 1.4 g of the title compound. 1H NMR (CDCl3) δ1.52 (s, IH), 2.57 (s, 3H), 4.72 (d, 2H), 7.32 (d, IH) , 7.49 (d, IH), 7.62 (s, IH).
Step 3, 3-cyano-4-methylbenzaldehyde
Treat 3-cyano-4-methylbenzyl alcohol (1.4 g, 10 mmol) in chloroform (30 mL) with manganese dioxide (3.3 g, 40 mmol). The reaction mixture was stirred at room temperature for 12 hours, filtered, the solvent was evaporated and the crude residue was purified by flash chromatography on silica gel, eluting with ethyl acetate:hexane (5:95) to give 0.9 g of the title compound. 1H NMR (CDCl3) δ2.67 (s, 3H), 7.48 (dd, IH), 8.0 (dd, IH), 10.0 (s, IH) .
References
[1] Yang, Lu, Wei, et al. Redox neutral [4+2] benzannulation of dienals and tertiary enaminones for benzaldehyde synthesis[J]. Chemical Communications, 2018.
[2] From PCT Int. Appl., 2000078768, 28 Dec 2000

 微信扫一扫打赏
微信扫一扫打赏

