Background and Application[1]
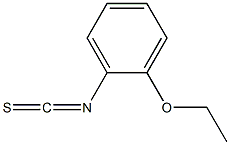
Isothiocyanate compounds, including 2-ethoxyphenyl isothiocyanate, are a very important functional substance in natural products and pharmaceutically active compounds. They are a class of compounds with an R-N=C=S structure. The compounds of the general formula are an important class of organic synthesis intermediates and are widely used in mineral processing, medicine, pesticides and dyes.
It is used in medicine for antibacterial, anti-inflammatory and preventive treatment of cancer diseases; in agriculture it can be used as an antibacterial agent, insecticide, herbicide, etc. In addition, isothiocyanates can also participate in a variety of organic reactions and are used to synthesize various types of compounds containing sulfur, nitrogen, and oxygen, especially heterocyclic compounds, and are widely used in organic synthesis products such as pesticides, medicines, and dyes. preparation. It can also be used to determine the sequence of amino acids in peptides and proteins and as a fluorescein label. Cruciferous vegetables containing isothiocyanates have been studied for four decades in cancer chemoprevention. More than 20 epidemiological study reports covering lung cancer, breast cancer, prostate cancer, bladder cancer, gastric cancer, colorectal cancer and kidney cancer have shown that the intake of cruciferous vegetables containing isothiocyanate compounds is related to The risk of multiple cancer prevalence is inversely related. At present, the application of isothiocyanate compounds in the prevention of lung cancer, prostatic hyperplasia and oral cancer in healthy people has been approved by the United States or China to enter the clinical stage.
Preparation[1]
2-Ethoxyphenyl isothiocyanate is prepared as follows:
Put 4-ethoxyaniline (2.19mol) and 309.2g triethylamine (3.06mol) into a 5000mL three-necked round-bottomed flask, add 1000mL ethyl acetate, cool in an ice bath and stir mechanically. When the temperature of the mixed liquid drops to 0℃~5℃, use a constant pressure dropping funnel to slowly add 299.2g CS2 (3.93mol) dropwise, stir magnetically while adding, and control the temperature of the reaction liquid at below 30 ℃. After the dropwise addition is completed, stir magnetically at room temperature (10-30 degrees range) for one hour; at this temperature, add 299.2g CS2 (3.93mol) and DMAP catalyst to the reaction solution, stir at 30± The reaction was stirred at 2.5℃ for 1.5h. After the reaction is completed, the reaction solution is washed twice with acid solution. The total amount of acid consumed in washing is 1.5 times that of triethylamine, and then washed with saturated brine until neutral. The organic phase was concentrated by rotary evaporation to obtain 2-ethoxyphenyl isothiocyanate.
References
[1]CN201710108328.9 A method for preparing high-purity isothiocyanate compounds suitable for industrial production

 微信扫一扫打赏
微信扫一扫打赏

