Background and overview[1]
Ethoxyformylethyl triphenylphosphine bromide is an organic intermediate that can be prepared in one step from triphenylphosphine and ethyl 2-bromopropionate. Ethoxyformylethyltriphenylphosphine bromide can be used to prepare 2-C-methyl-4,5-O-(1-methylvinyl)-D-arabinonate ethyl ester, 2-C-methyl Ethyl-4,5-O-(1-methylvinyl)-D-arabinonate is a key intermediate in the synthesis of sofosbuvir.
Preparation[1]
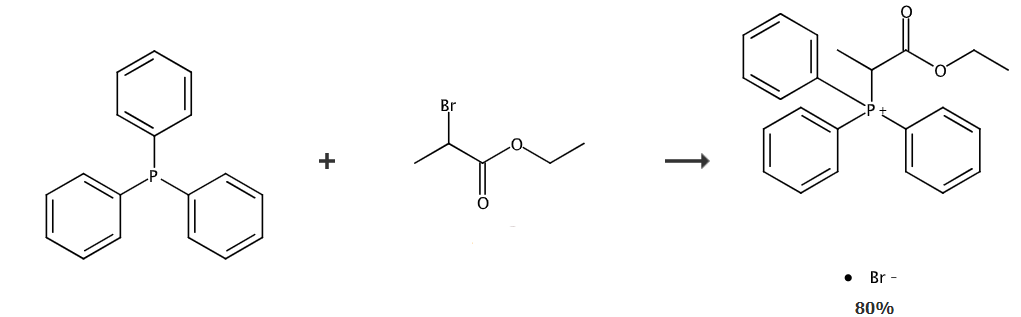
Add 28.8g (0.11mol) triphenylphosphine and 18.1g (0.1mol) ethyl 2-bromopropionate into 157mL water, raise the temperature to 70~80°C, and continue the insulation reaction at this temperature. After 15 to 18 hours, an ethoxyformylethyltriphenylphosphine bromide solution was obtained.
Apply[1]
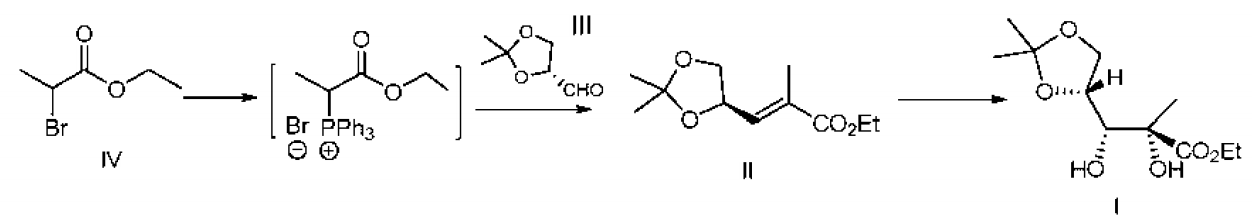
The method for preparing 2-C-methyl-4,5-O-(1-methylvinyl)-D-arabinonate ethyl ester from ethoxyformylethyltriphenylphosphine bromide is as follows: Add 34.0g (0.13mol) triphenylphosphine and 23.5g (0.13mol) 2-bromopropionic acid ethyl ester to 157mL of water, raise the temperature to 70~80°C, and continue the insulation reaction at this temperature for 15~18 hours , lower the temperature of the reaction system to 0~5°C, add 200ml acetonitrile, and dropwise add 26g of acetonitrile solution containing 13.0g (0.1mol) R-glyceraldehyde acetal. The dropwise addition is completed in about 0.5 hours, and continue to add 30% hydrogen dropwise. 20.5g (0.11mol) of potassium oxide solution, control the internal temperature not to exceed 10°C, complete the dropwise addition, raise the temperature to 20-25°C and keep stirring for 1-3 hours until the reaction of R-glyceraldehyde acetone is completed. Add 7.5 mg of potassium osmate (2.0 × 10 mol) to the system, stir at room temperature, add 74 g of 15% potassium ferricyanide aqueous solution in batches, and stir at room temperature for 24 hours. After the reaction is complete, quench the reaction with saturated sodium sulfite aqueous solution and separate the liquids. , the aqueous phase is extracted with dichloromethane solution, the organic phases are combined, concentrated, and then a mixed solvent of 40 ml of toluene and 100 ml of n-heptane is added to the concentrated system, heated and refluxed until completely dissolved, cooled to 0-5°C and stirred After 1 hour, suction filtration was performed to obtain 14.7g of 2-C-methyl-4,5-O-(1-methylvinyl)-D-arabinonate ethyl ester (purity 96.9%, yield 64.1%).
References
[1] [Chinese invention] CN201710542417.4 Preparation method of 2-C-methyl-4,5-O-(1-methylvinyl)-D-arabinonate ethyl ester

 微信扫一扫打赏
微信扫一扫打赏

