Background and overview[1]
(4-Chlorophenyl)glyoxylic acid is a pharmaceutical intermediate that can be prepared from chlorobenzene and ethyl 2-chloro-2-oxoacetate in two steps. It can be used to prepare a drug that can be used to treat and Compounds for KIT and PDGFR-related disorders
Preparation[1]
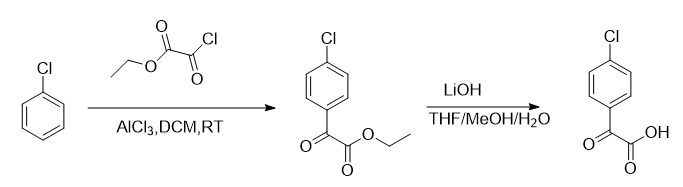
Step 1: Synthesis of ethyl 2-(4-chlorophenyl)-2-oxoacetate
To a stirred solution of chlorobenzene (10 mL, 100.0 mmol) and ethyl 2-chloro-2-oxoacetate (17 mL, 146.0 mmol) in DCM (250 mL) at 0 °C was slowly added aluminum trichloride (25.5g, 192.0mmol). After the addition was complete, the reaction was allowed to warm to room temperature and stirred for an additional 12 hours. The reaction was diluted with EtOAc (500 mL) and washed with water (300 mL) and brine (300 mL). The organic layer was separated, dried over Na2SO4, filtered and concentrated. The residue was purified by silica gel column chromatography (PE:EtOAc=10:1 to 5:1) to obtain the title compound (14 g, 67%) as a light oil. MS(ES+)C10H9ClO3 required value: 212,214, measured value: 213,215[M+H]+.
Step 2: Synthesis of (4-chlorophenyl)glyoxylic acid
Dissolve ethyl 2-(4-chlorophenyl)-2-oxoacetate (4.0g, 18.9mmol) and lithium hydroxide (1.98g, 47.1mmol) in THF:MeOH:H2The mixture in O (76 mL, v/v/v=10:6:3) was stirred at 25°C for 3 hours. The mixture was neutralized with concentrated HCl to pH=2-3. The white precipitate formed was collected via filtration, washed three times with water and dried under vacuum to give (4-chlorophenyl)glyoxylic acid (3.0 g, 86%) as a white solid. MS(ES+)C8H5ClO3 required value: 184,186, measured value: 185,187[M+H]+.
Apply[1]
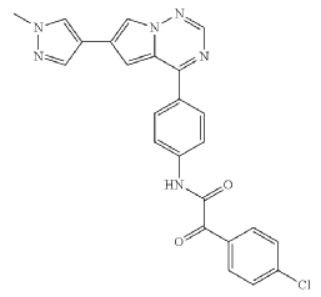
CN201680052913.5 reported that (4-chlorophenyl)glyoxylic acid was used to synthesize the above compound 2-(4-chlorophenyl)-2-hydroxy-N-(4-(6-(1-methyl) -1H-Pyrazol-4-yl)pyrrolo[1,2-f][1,2,4]triazin-4-yl)phenyl)acetamide, used to treat diseases such as mastocytosis, GIST and AML disease compounds. Such compounds can be used to treat human or non-human diseases related to abnormal KIT activity. Activating mutations in KIT can be found in a variety of indications, including systemic mastocytosis, GIST (gastrointestinal stromal tumor), AML (acute myeloid leukemia), melanoma, seminoma, intracranial germ cell tumors and mediastinal B-cell lymphoma.
References
[1] [Invented in China] CN201680052913.5 Compounds useful for treating conditions related to KIT and PDGFR

 微信扫一扫打赏
微信扫一扫打赏

