Background and overview[1]
1,3,5-Tris(4′-carboxy[1,1′-biphenyl]-4-yl)benzene belongs to the polybiphenyl polyacid monomer and is a metal-organic framework material (MOF) Or organic self-assembly materials, which are a popular field of modern materials chemistry. Because they are safer and have more storage capacity than ordinary cylinders for storing clean energy (hydrogen, methane), they are the future new energy vehicles (fuel cell vehicles, etc.) important basis for development.
Preparation[2]
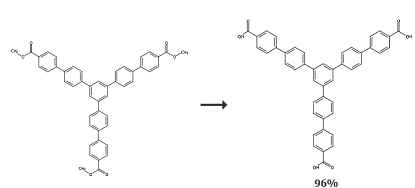
1,3,5-Tris(4′-carboxy[1,1′-biphenyl]-4-yl)benzene was placed in a 1 L round-bottomed flask equipped with a magnetic stirring rod. 4″-(Benzene-1,3,5-triyl-tris(phenyl-4,1-diyl)benzene)tribenzoate (2.00 g, 2.82 mmol), 2.2 g NaOH, THF, water and Methanol (200, 80 and 50 mL respectively) were mixed and the mixture was stirred at room temperature for 2 days. The solution was evaporated under vacuum. Acidified with 3 M HCl. The product was collected by filtration as a white solid and treated with dichloromethane and methanol. Wash, then vacuum dry overnight (1.80 g, 96%): mp 326 °C. 1H-NMR (DMSO-d6, 400 MHz) δ 13.07 (br, 3H) 8.07 (d, 6H, J = 8.0Hz), 8.06 (s, 3H), 8.05 (d, 6H, J = 8.0Hz), 7.91 (d, 6H, J = 7.6Hz), 7.89 (d, 6H, J = 7.2Hz); 13C-NMR (DMSO-d6, 100 MHz) δ168.0, 144.6, 141.9, 140.7, 139.2, 130.9, 130.6, 128.8, 128.4, 127.6, 125.3; FT-IR (KBr, 4000- 400 cm ), 1276 (m), 1177 (m), 1118 (m), 1003 (m), 824 (s), 773 (s); MALDI-TOF MS (m/z) [M] + 666.2976, 666.2042 (calculated).
Main reference materials
[1] [Chinese invention] CN201610671354.8 A method for preparing polybiphenyl polyacid monomer
[2] From PCT Int. Appl., 2011038208, 31 Mar 2011

 微信扫一扫打赏
微信扫一扫打赏

