Background and overview[1-2]
2-Methoxy-4-nitrobenzaldehyde is an organic intermediate. There are two main synthesis routes for its preparation. One is prepared in three steps using the compound 4-nitrosalicylic acid as the starting material, and the other is prepared in two steps using 4-nitro-2-methoxytoluene as the starting material.
Preparation[1-2]
Report 1,
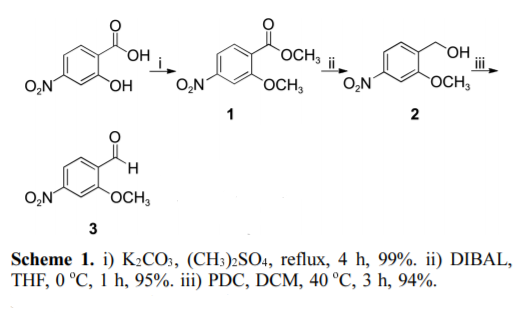
4-Nitrosalicylic acid is esterified by dimethyl sulfate, then reduced by DIBAL-H to obtain 2-methoxy-4-nitrobenzyl ethanol, and finally oxidized by PDC to obtain 2-methoxy-4 -Nitrobenzaldehyde.
Report 2,
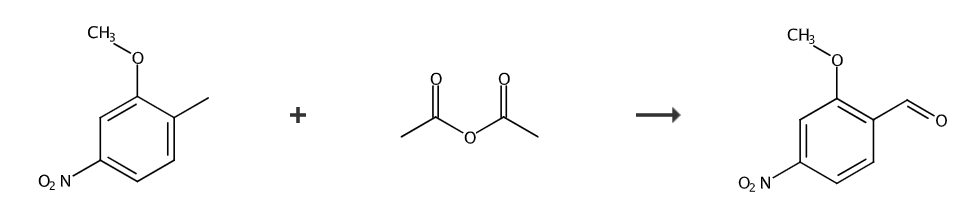
The first step, preparation of 4-nitro-2-methoxy-(α,α-diacetoxy)toluene:
Add 4-nitro-2-methoxytoluene (150.0g, 0.8973mol), HOAc (900mL) and Ac2O (900mL) into a 5L three-neck round-bottom flask equipped with a mechanical stirrer. The mixture was stirred and cooled to 8°C using an acetone/ice bath. Carefully add concentrated H2SO4 (136 mL) while keeping the reaction temperature below 19°C. After cooling to 0°C, CrO3 (252.6g, 2.526mol, 2.815 equivalents) was added in batches within 1 hour while maintaining the reaction temperature between 0-10°C. After the addition was complete, the mixture was stirred at 0°C for 30 minutes, at which time the reaction was complete. The reaction mixture was then carefully poured into ice (1.5 kg) with stirring to obtain a slurry. The remaining black gummy residue was rinsed with HOAc (3x100mL) and the wash solution was added to the slurry. After stirring for 10 minutes, the slurry was filtered. The filter cake was washed with water (3 × 400 mL) and dried under vacuum for 17 hours to obtain compound 4-nitro-2-methoxy-(α,α-diacetoxy)toluene (129.0 g, 51%).
Second step, preparation of 2-methoxy-4-nitrobenzaldehyde:
Place compound 4-nitro-2-methoxy-(α,α-diacetoxy)toluene (250.7g, 0.8851mol) into a 2L round-bottomed flask equipped with a condenser and a mechanical stirrer. dioxane (300 mL) and concentrated HCl (60 mL). The reaction mixture was heated to reflux and stirred under N2 for 20 hours. Water (250 mL) was added dropwise while keeping the reaction mixture at reflux. After cooling to 0°C with an ice/water bath, the resulting slurry was stirred for 30 minutes and then filtered. The filter cake was washed with water (4 × 200 mL) and dried under vacuum for 17 hours to obtain the compound 2-methoxy-4-nitrobenzaldehyde (146.3 g, 91%) as a yellow solid.
References
[1]Synthesis of green emitting coumarin bioconjugate for the selective determination off luantigen, ByBhusal,RamPrasadetal,FromBulletinoftheKoreanChemicalSociety,32(5),1461-1462;2011
[2]FromPCTInt.Appl.,2004000214,31Dec2003

 微信扫一扫打赏
微信扫一扫打赏

