Background and overview[1]
2-Trifluoromethylbenzonitrile can be used as a pharmaceutical synthesis intermediate and can be prepared by reacting 2-trifluoromethylbenzaldehyde as raw material and hydroxylamine hydrochloride.
Preparation[1-2]
Report 1,
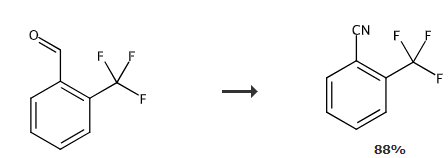
Combine 2-trifluoromethylbenzaldehyde (2.0mmol, 1.0 equivalent), H2NOH·HCl (153mg, 2.2mmol), Na2CO3 (117 mg, 1.1 mmol) and DMSO (10 mL, 0.2 M) were added to an oven-dried reaction tube (30 mL) equipped with a stir bar. The reaction mixture was allowed to react at room temperature for 30-60 minutes and the reaction was monitored by TLC. After the aldehyde is completely consumed, add another portion of Na2CO3 (1.06g, 10mmol) to the mixture and cover the reaction tube with a plastic stopper. The SO2F2 gas was passed through the SO2F2 balloon and stirred with slow bubbling at room temperature for 12 hours. The reaction mixture was diluted with water, and the mixture was extracted with dichloromethane (3 × 20 mL). The combined organic layers were washed with brine and anhydrous Na2< Dry the mixture over SO4 and concentrate the mixture to dryness. The residue was purified by silica gel chromatography using a mixture of petroleum ether/ethyl acetate = 10:1 (v/v) as the eluent to obtain 2-trifluoromethylbenzonitrile.
Report 2,
Place 2-trifluoromethyliodobenzene (0.50mmol), [(2,2′-bipyridine)2Cu][O2CCF 2Cl] (631 mg, 1.25 mmol, 2.5 equiv), NaOH (60 mg, 1.5 mmol), CsF (228 mg, 1.50 mmol)) and DMF (6 mL) were added to a Teflon screw cap equipped with a stirring rod. reaction tube, seal the test tube, and place the solution in a preheated 75°C oil bath for 3 hours. Remove the test tube from the oil bath and cool to room temperature. Add (trifluoromethoxy)benzene (65 mL, 0.50 mmol) as an internal standard. The reaction mixture was filtered through a celite layer to obtain 2-trifluoromethylbenzonitrile.
References
[1] Cascade Process for Direct Transformation of Aldehydes (RCHO) to Nitriles (RCN) Using Inorganic Reagents NH2OH/Na2CO3/SO2F2 in DMSO
[2] Trifluoromethylation of (hetero)aryl iodides and bromides with copper(I) chlorodifluoroacetate complexes

 微信扫一扫打赏
微信扫一扫打赏

