Background and overview[1]
3-Chloro-2-hydroxybenzene can be used as an intermediate for pharmaceutical and chemical synthesis. If 3-chloro-2-hydroxybenzene is inhaled, move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; if the eyes are clear In case of contact, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Structure[1, 3]
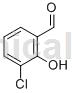
Preparation [1]
To a stirred solution of 2-chlorophenol (20.0g, 155.60mmol) in acetonitrile (200mL) at room temperature were added MgCl 2 (22.2g, 233.35mmol) and triethylamine (59.03g, 583.39mmol). Paraformaldehyde, 0.52 g, 1.05 mol) was added to the mixture and then refluxed for 4 hours. The resulting mixture was cooled to room temperature, quenched with 2N HCl, extracted with diethyl ether, dried over MgSO4, filtered and concentrated under reduced pressure. The crude compound was purified by silica gel column chromatography to obtain the title compound 3-chloro-2-hydroxybenzene. (16.7 g, 60-80%) 1 H NMR (400MHz, CDCl 3) δ 7.01 (t, J = 7.8, 1H), 7.49-7.56 (m, 1H), 7.63 (d, .7 = 8.0, 1H ), 9.91 (s, 1H), 11.50 (s), 1H).
Apply[1]
3-Chloro-2-hydroxybenzene can be used as an intermediate for pharmaceutical and chemical synthesis. For example, the following compounds are synthesized:
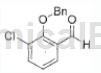
The specific steps are as follows: to the stirring solution of 3-chloro-2-hydroxybenzene (16.5g, 106.98mmol) in acetonitrile (165mL), add benzyl bromide (19.21g, 112.33mmol) and K2CO3 (17.74g, 128.38mmol), room temperature order. The mixture was heated to reflux and stirred for 4 hours. The resulting mixture was cooled to room temperature, filtered through Celite, and concentrated under reduced pressure. The crude compound was purified by silica gel column to obtain the title compound. (22.4g, 80~95%).
1 H NMR (400MHz, CDCl 3) δ5.16 (s, 2H), 7.18-7.23 (m, 1H), 7.35-7.45 (m, 5H), 7.66-7.71 (m, 2H), 7.71- 7.76 (m, 1H), 10.12 (d, .7 = 0.8, 1H).
Main reference materials
[1] WO2015088272 SULFAMATE DERIVATIVE COMPOUNDS FOR USE IN TREATING OR ALLEVIATING PAIN

 微信扫一扫打赏
微信扫一扫打赏

