Background and overview[1][2]
Adamantane (tricyclo[3.3.1.1]decane) is a symmetrical, very stable cage hydrocarbon, a cyclic tetrahedral hydrocarbon composed of 10 carbon atoms and 16 hydrogen atoms. The basic skeleton is similar to a lattice unit of diamond. Within its molecular weight range, adamantane is the most spherical hydrocarbon molecule among known molecules. Its rigid ring system and symmetrical structure determine that it has unique physical characteristics and chemical properties.
Adamantylphenol, also known as 4-(1-adamantyl)phenol, can be used as a monomer for the synthesis of highly heat-resistant polyarylates, polyarylene ethers, and functional polymers such as polyarylsulfones. It can also be used as Other additives are used. Adamantane has shown broad application prospects in high-tech fields such as microelectronics, communications, aviation, optical instruments, and biomedicine. There have been no reports on the synthesis method of 4-(1-adamantyl)phenol.
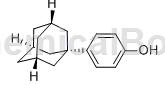
4-(1-adamantyl)phenol
Apply[1]
Based on the compound 4-(1-adamantyl)phenol, a new type of 4-(1-adamantyl)phenol derivatives containing adamantane structural units is formed by structural modification of its phenol ring. Ability to inhibit hypoxia-inducible factor-1. This class of compounds has a significant inhibitory effect on hypoxia-inducible factor-1.
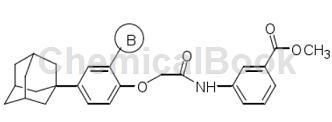
Preparation[2]
Put 32g (0.235mol) adamantane and 12ml of 0.235mol liquid bromine into a 100ml reaction bottle equipped with a hydrogen bromide absorption device, raise the temperature to 50°C, react for 0.5 hours, react at 90°C for 1.5 hours, and then heat at 120 ℃, react for 2 hours. Leave overnight. Use saturated sodium bisulfite aqueous solution to reduce unreacted bromine, filter, wash with water until neutral, and dry in a vacuum oven. Recrystallize with methanol to obtain light yellow crystals, which are dried and the yield is 71.1%.
Put 10g of dried 1-bromoadamantane (0.047mol), 5.2g of phenol (0.047mol), and 50ml of benzene (0.56mol) into a 100ml reaction bottle connected to a reflux device. Reaction at 40°C for 6 hours, reaction at 60°C for 24 hours, and reflux reaction at 90°C for 42 hours. The solution after the reaction is treated with hot water to precipitate 4-(1-adamantyl)phenol, while washing away unreacted resorcinol and filtering to obtain the product. The product is dried in a vacuum drying oven and then recrystallized with toluene. A white product was obtained with a yield of 67.5%. The above-mentioned 4-(1-adamantyl)phenol was synthesized.
Main reference materials
[1] Duan Qiong. (2016). Synthesis and anti-tumor activity of a new adamantane-containing hypoxia-inducible factor-1 inhibitor. (Doctoral dissertation, Changchun University of Technology).
[2] Liu San, & Guo Jianwei. (2008). Synthesis of new adamantyl polyarylates by phase transfer catalysis method. Acta Chemical Engineering, 59(6), 1556-1564.

 微信扫一扫打赏
微信扫一扫打赏

