Background and overview[1]
9,9-Diphenylfluorene-2-boronic acid is an organic acid compound that can be used as an intermediate in organic synthesis. If 9,9-diphenylfluorene-2-boronic acid is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel unwell. ; If eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
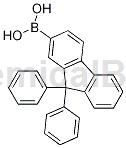
Structure
Apply[1]
9,9-Diphenylfluoren-2-boronic acid can be used as an intermediate in organic synthesis, such as the synthesis of 2-chloro-4-(9,9-diphenylfluoren-2-yl)quinazoline, specific steps They are as follows: 20 grams of 2,4-Dichloro-quinazoline, 40 grams of 9,9-diphenylfluorene-2-boronic acid, 5.8 grams of tetrakis(triphenylphosphorus)palladium ( A mixture of triphenyl-phosphine palladium, 300 ml of toluene, 44 ml of ethanol and 130 ml of 2M aqueous solution of potassium carbonate was added to a 500 ml flask and refluxed for 16 hours. After quenching the reaction with water, the organic layer was washed with water to remove the alkali residue. The organic layer was separated, dried over anhydrous sodium sulfate, concentrated, and precipitated with methanol to obtain a white solid 2-chloro-4-(9 ,9-Diphenylfluoren-2-yl)quinazoline 25 grams.
Preparation[1]
The preparation of 9,9-diphenylfluorene-2-boronic acid is as follows: Take 10 grams of 2-bromo-9,9-diphenylfluorene and 100 ml of anhydrous tetrahydrofuran, add it to a 1-liter long-neck flask, and add liquid nitrogen to Cool to -78°C, slowly add 25.2 ml of n-butyllithium and trimethyl borate successively while stirring. After the addition is completed, return to room temperature naturally, add an appropriate amount of dilute hydrochloric acid for hydrolysis, and then extract with ethyl acetate and separate. After the organic layer was removed, it was dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure, and 4 g of the product 9,9-diphenylfluorene-2-boronic acid was precipitated with hexane.
Main reference materials
[1] CN201610812783.2 Organic electroluminescent compounds and organic electroluminescent components

 微信扫一扫打赏
微信扫一扫打赏

