Background and overview[1]
Tert-butyl ester is a class of compounds with important structural units because it is widely used in the field of synthesis and is used in the synthesis of drugs and agricultural chemicals. In addition, tert-butyl ester also has a very important advantage as a protecting group and source of carboxylic acid. Therefore, the synthesis of tert-butyl ester compounds has always attracted the attention of pharmacologists and chemists.
tert-Butyl 4-methylbenzoate can be used as an intermediate in organic synthesis. The traditional synthesis method of tert-butyl ester compounds is mainly through the reaction of carboxylic acid or its derivatives with tert-butyl alcohol. However, harsh reaction conditions and acid corrosion of instruments limit the further application of this method. In recent years, many literatures have reported on the synthesis of tert-butyl ester compounds, such as the synthesis of tert-butyl ester by carbonyl insertion of halobenzene and potassium tert-butoxide; the synthesis of tert-butyl ester by C-H activation catalyzed by transition metal Ru, and the synthesis of tert-butyl ester by catalyzing benzyl by transition metal Ir Alcohols and methyl tert-butyl esters are used to synthesize new tert-butyl ester compounds. Although these methods can be used to effectively synthesize tert-butyl ester compounds, these methods have certain limitations: the use of a large amount of acid affects the instrument. Certain corrosion effects; the use of expensive transition metals; higher reaction temperatures; the use of halogens will inevitably pollute the environment. Therefore, developing an efficient method for synthesizing tert-butyl ester compounds under mild reaction conditions without metal catalysis is one of the issues that organic chemists urgently need to solve.
Structure
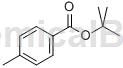
Synthesis method[1]
The synthesis of tert-butyl 4-methylbenzoate is as follows: add 0.2mmol p-methylbenzonitrile, 50mgMgSO4, 0.5mmol tert-butyl peroxide into the reaction vessel, and then add 2 ml acetonitrile, react at 50°C. After the reaction is completed, wash with water, then extract with an organic solvent, dry, distill under reduced pressure and concentrate to remove the solvent. The crude product is separated by column chromatography to obtain the target product 4-methylbenzoic acid tert-butyl ester. Yield 77%.
Main reference materials
[1] CN201610316226.1 A synthesis method of tert-butyl ester compounds

 微信扫一扫打赏
微信扫一扫打赏

