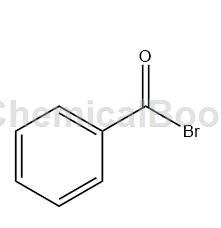
2-Bromobenzophenone can be used as an intermediate in organic synthesis and pharmaceutical research and development, and is suitable for laboratory organic synthesis and chemical and pharmaceutical synthesis processes.
Apply[1]
1. Preparation of N-benzoylmethylpyridine bromide
Add 1200mL dichloromethane, add 4mol pyridine (316g), react at 30°C for 15 hours, the reaction is completed, cool to room temperature, add 600mL water and 800mL ethyl acetate for extraction, separate the organic phase, and use ethyl acetate for the water phase Extract (3 × 600 mL), combine the organic phases, wash with water (2 × 250 mL), wash with 200 mL of saturated brine, dry with anhydrous sodium sulfate, filter, and concentrate the filtrate to obtain N-benzoylmethylpyridine bromide crude product, which The crude product was recrystallized from a mixed solution of n-hexane: ethyl acetate = 1:2 (volume ratio) to obtain 107.1g of pure product, with a yield of 64.2%.
2. Unsubstituted thiosemicarbazone thiazole
Add 0.1035g of the unsubstituted ketone thiosemicarbazone and 0.8mmol of 2-bromobenzophenone into a 100ml round-bottomed flask, and take 20ml of absolute ethanol as the solvent. Add the rotor, heat, stir and reflux in a constant temperature water bath at 80°C for two hours. The solution changes from colorless to yellow, and a small amount of solid precipitates at the bottom. After standing for a while, a large amount of solid will precipitate. Filter and dry at room temperature to obtain 0.048g of yellow solid, with a yield of 35.58%.
Preparation[2]
Dissolve 600mmol (72.0g) benzophenone in 500mL methylene chloride, add 2mol liquid bromine (320g) dropwise at 0°C, and stir for 3 hours after the dripping. At the end of the reaction, the solvent was distilled off under reduced pressure to obtain 2-bromobenzophenone.
Main reference materials
[1] Dai Liyan, & Chen Yingqi. (2000). Synthesis of 2-amino-4′-bromo-benzophenone. Chemical Bulletin, 63(5), 51-52.
[2]Geng Xuanping, Zhang Xianglong, Cheng Wei, Han Meng, Lai Xinsheng, & Lai Chao, et al. (0). A method for the preparation of N-benzoylmethylpyridine bromide.

 微信扫一扫打赏
微信扫一扫打赏

