Background and overview[1][2]
Toluidine blue is also called toluidine blue; 2,2′-[(9,10-dihydro-4,8-dihydroxy-9,10-dioxo-1,5-anthracenediyl)di Imino]bis(5-methylbenzenesulfonic acid) disodium salt; toluidine blue/toluidine blue; toluidine blue O.
Toluidine blue is a photosensitizer gel, which has the following major advantages: The gel preparation has a certain degree of viscosity and adhesion, which allows the drug to stay in the affected area to improve the therapeutic effect. Toluidine blue, on the other hand, is a nucleic acid-prone eosinophilic dye that stays in tissues with a large nuclear component. It has good penetration depth in tissues and can be effectively distributed in infected target tissues, but it cannot penetrate into normal tissues. This prevents normal tissues from being damaged by photodynamic forces. Therefore, toluidine blue is effective against diseased areas and bacteria. Has better selectivity. The most important point is that toluidine blue photosensitizer has no obvious toxic and side effects, which reduces the harm to human health systems and is conducive to the promotion and application of photodynamic therapy.
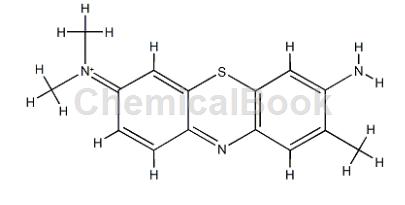
Toluidine blue
Apply[3]
Multi-functional rapid dye for thoracic and ascites effusion and gynecological exfoliated cells
Composition of dye liquor:
Recipe: Ingredients: Thionine, Toluidine Blue, Trisodium Phosphate, Water
The ratio is 1:2:1:10
Preparation method: Mix thionine, toluidine blue and water, shake well and then add trisodium phosphate to mix and filter the dye solution.
How to use: After the smear of various exfoliated cells is slightly dry, add 3-4 drops of this dye to fully cover the smear surface, rinse it with running water after about 30 seconds to 1 minute, and then wet the smear. Microscopic examination. For those who need an oil microscope, wait for the smear to dry and then add cedar oil for microscopic examination.
Advantages: Compared with traditional Gram’s and Wright’s stains and existing multi-functional stain detection reagents for gynecological leucorrhea smears, the advantages are as follows:
First, the background of the cells stained by this dye on the same smear is very clear. The nuclei and plasma are stained at once, and the red and blue are distinct. The various stained exfoliated cells, bacteria, and fungi are bright in color and clear in shape, just like a smear. Color pictures; however, the background produced by traditional staining solutions is often unclear, the nucleus and pulp are blurred, and the staining procedures are complicated; whether it is a traditional staining solution or a multi-functional staining solution for gynecological leucorrhea smear, they are both necessary for the nucleus and pulp. Dye in two steps; first dye the nucleus first, then dye the pulp well the second time; and do not use one solution for multiple uses.
Secondly, the positive rate of the same patient and the same smear stained with this dye is 60% higher than the positive rate of traditional staining; it is higher than the 26% positive rate of multi-functional staining of gynecological leucorrhea smears;
p>
Preparation[2]
1. Synthesis of indamine
Add 5g of N,N-dimethyl-p-phenylenediamine dihydrochloride powder to 155g of USP (United States Pharmacopoeia) pure water in a round-bottomed flask, and stir at 300 rpm. The N,N-dimethyl-p-phenylenediamine reaction mixture 20 should be maintained at ≤10°C and stirred for 10 minutes.
O-Toluidine Hydrochloride was prepared by slowly adding 6.3 g of hydrochloric acid (6N) to 2.8 g of o-toluidine. The o-toluidine hydrochloride solution 22 should be stirred until clear. If crystals appear, a minimum amount of USP pure water should be added to redissolve the o-toluidine hydrochloride crystals.
Add o-toluidine hydrochloride solution 22 to N, N-dimethyl-p-phenylenediamine dihydrochloride reaction mixture 20, and then add 2g HCl (6N) 24. The reaction mixture should be maintained below about 10°C and stirred on an ice bath for 45 minutes.
Use a dropping funnel to slowly drop (over 20 minutes) 29.25g of dichromate solution 28 into the reaction mixture 26 of o-toluidine hydrochloride and N,N-dimethyl-p-phenylenediamine dihydrochloride, so The dichromate solution 28 was prepared by adding 9.39 g of potassium dichromate to 108 g of USP pure water. Once all dichromate solution 28 has been added, the indamine dihydrochloride reaction mixture 30 should be maintained below about 10°C and stirred on an ice bath for 60 minutes. Those skilled in the art will understand that the reaction mixture includes indamine dihydrochloride and its derivatives.
2.Synthesis of S-indalaminothiosulfate
Aluminum sulfate solution 32 was prepared by adding 8.75g of aluminum sulfate hexahydrate to 15g of USP pure water to obtain the acid. The acid was added to the indamine dihydrochloride reaction mixture 30 and stirred for 10 minutes. Acids other than aluminum sulfate hexahydrate are also suitable.
Zinc chloride solution 34 was prepared by adding 12.22g of zinc chloride to 15g of USP pure water, and then the zinc chloride solution 34 was used to obtain a complexing agent. This complexing agent was added to the indamine dihydrochloride reaction mixture 30 and stirred for 10 minutes. It will be understood that although the preferred embodiments include zinc chloride, other complexing agents are suitable and included in the present invention.
Dichromate solution 36 was prepared by adding 9.39 g of potassium dichromate to 108 g of USP pure water, and 29.25 g of the dichromate solution 36 was further prepared to obtain an oxidizing agent. The oxidant was added slowly (over 20 minutes) to the indamine dihydrochloride reaction mixture 30 using a dropping funnel and stirred on an ice bath for 20 minutes.
Prepare sodium thiosulfate solution 38 by adding 6.53g of sodium thiosulfate pentahydrate to 15g of USP pure water to obtain a sulfur-containing nucleophile source. This sulfur-containing nucleophile source was slowly added to the indamine dihydrochloride reaction mixture 30 and stirred on an ice bath for 60 minutes. The precipitate formed contains S-indaminylthiosulfate 40 and its derivatives. Technology in this fieldPersonnel should understand that other sources of sulfur-containing nucleophiles may be used instead of sodium thiosulfate solution38.
3. Synthesis of toluidine blue and toluidine blue zinc double salt
Maintain S-indaminothiosulfate 40 below 10°C. Add the oxidizing agent slowly (over 20 minutes) to S-indaminothiosulfate 40 using a dropping funnel and stir for 20 minutes. It is currently believed that 29.25 g of dichromate solution 42 is suitable for use as oxidant.
Add a complexing agent, preferably 27.0 g of zinc chloride solution 46, to the oxidized S-indaminyl thiosulfate reaction mixture 44, and stir for 5 minutes. The zinc chloride solution 46 passes Prepare by adding 12.22g zinc chloride to 15g USP pure water. Add an oxidation catalyst, preferably 17.3 g of copper sulfate solution 48 prepared by adding 2.38 g of copper sulfate to 15 g of USP pure water, and stir for 5 minutes.
Change the temperature set point to 60°C. Once the reaction reaches 60°C, an acid, preferably sulfuric acid solution 50 (9N), is added to lower the pH to 2.9. Stir for 5 minutes after each addition of acid. Change the temperature set point to 97°C. Once the reaction mixture reaches 97°C, stir for 35 minutes. Reaction mixture 52 was slowly cooled to room temperature. After reaching room temperature, the product containing crude toluidine blue product mixture 54 was placed in a 5°C cooler. Store for 5 to 15 hours. Remove and filter a portion of the crude toluidine blue product mixture 54 through a 0.45 μm filter. The filtered solution was placed into vials for analysis by the RP-HPLC (reverse phase high performance liquid chromatography) toluidine blue analytical method.
Main reference materials
[1] Chu Qinghui. (1997). Electrochemical behavior of toluidine blue. Analytical Chemistry (7), 829-831.
[2] Dagula, Cao Gaowa, & Zhang Li. (2004). Determination of cr(Ⅵ) by toluidine blue fading photometry. Journal of Inner Mongolia University for Nationalities (Natural Science Edition), 19(5), 516-517.
[3] Liang Rui. (2006). Cervical smear staining with toluidine blue dye. Journal of Shanxi College of Traditional Chinese Medicine, 7(1), 27-27.

 微信扫一扫打赏
微信扫一扫打赏

