Background and overview[1]
Phenylhydrazine was first synthesized by Hermann Emil Fischer in 1875 and was the first hydrazine derivative synthesized. With the deepening of research on heterocyclic compounds and the continuous expansion of applications, substituted phenylhydrazine compounds have a wide range of applications. In medicine, they can synthesize new antibacterial drugs, treat diabetes, anticancer drugs, antiviral drugs, antihypertensive drugs, etc. In terms of pesticides, it can synthesize insecticides, bactericides and herbicides, etc. It is also widely used in the fuel industry, charge transfer materials, polymers and other industries.
The production process of substituted phenylhydrazine series compounds including 2,3-dichlorophenylhydrazine hydrochloride is similar, mainly the diazotization reaction of aromatic amines, and then reduction with sodium sulfite or stannous chloride to obtain various substitutions Phenylhydrazine compound. At the same time, phenylhydrazine salts and substituted phenylhydrazine salts (such as 2,3-dichlorophenylhydrazine hydrochloride, etc.) are important pharmaceutical and pesticide intermediates. So far, there is basically no integrated synthesis process for the industrial synthesis of high-purity products. With the vigorous development of the pharmaceutical, pesticide, and dye industries, the market demand for various substituted phenylhydrazine compounds will be increasing. Therefore, the development of a continuous production process for a series of substituted phenylhydrazine compounds has practical significance and great prospects
Apply[2-3]
2,3-Dichlorophenylhydrazine hydrochloride can be used as an intermediate for pharmaceutical and chemical synthesis, such as the preparation of the following compounds:
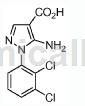
Step 1: Combine 2,3-dichlorophenylhydrazine hydrochloride (1g, 4.68mmol), (E)-ethyl 2-cyano-3-ethoxyacrylate (0.792g, 4.68mmol) and a solution of K2CO3 (0.647g, 4.68mmol) in EtOH (10mL) was added to a microwave vial and heated at 85°C for 20 hours. The reaction mixture was then cooled to room temperature and poured into ice water. The resulting suspension was then filtered, the solid was washed with water, and dried in a vacuum oven (50°C) for 4 hours to obtain a brown solid. The crude product was then purified by silica gel chromatography to obtain a brown solid as ethyl 5-amino-1-(2,3-dichlorophenyl)-1H-pyrazole-4-carboxylate (0.93 g, 66% yield ). MS (ESI) m/z: 300.0 (M+H)+.
Step 2: Dissolve the compound of Step 1 (0.026g, 0.087mmol) in MeOH (2mL), stir at room temperature, add 1.0N NaOH (0.260mL, 0.260mmol), and then heat to 70℃Keep it 24 hours. Additional 1 N NaOH (0.260 ml, 0.260 mmol) was added to the mixture, and the reaction mixture was warmed to 90°C for 7 hours. The reaction mixture was cooled to room temperature, 1N HCl (0.75 mL) was added, and the reaction mixture was concentrated to obtain a yellow solid as 5-amino-1-(2,3-dichlorophenyl)-1H-pyrazole-4-carboxylic acid. (0.065g, 99%). MS (ESI) m/z: 271.9 (M+H)+.
Main reference materials
[1] WO2018019250 A continuous flow synthesis process of phenylhydrazine salts and substituted phenylhydrazine salts
[2] WO2013022818) NOVEL MACROCYCLES AS FACTOR XIA INHIBITORS

 微信扫一扫打赏
微信扫一扫打赏

