background and overview[1-2]
chemiluminescence refers to the phenomenon of emitting visible light in some special chemical reactions. the luminescence mechanism is that certain substances in the reaction system absorb the energy released by the reaction and transition from the ground state to the excited state, and then return to the ground state from the excited state. the energy is released in the form of light radiation, producing luminescence. among many chemiluminescent reagents, luminol reagents have become the most widely used chemiluminescent reagents because they have high luminescence quantum yield and good water solubility, and can react chemically with a variety of oxidants. its luminescence mechanism is oxidation reaction luminescence. they are catalyzed by horseradish peroxidase under alkaline conditions and oxidized by hydrogen peroxide to produce an excited state intermediate that returns to aminoterephthalic acid. when it returns to the ground state, it emits photons. therefore, luminol reagents have good application value and broad market demand prospects.
apply[1]
luminol is a commonly used luminol-type chemiluminescent reagent. its luminescence mechanism is oxidation reaction luminescence. it is catalyzed by horseradish peroxidase under alkaline conditions and is oxidized by h2o2 to generate 4-amino group. the excited state intermediate of phthalic acid emits photons when it returns to the ground state.
preparation[1, 3]
method 1: a method for preparing high-yield isoluminol luminescent reagent, including the following steps:
(1) preparation of phthalimide: in the reactor, add 37.1g phthalic anhydride and 9.0g urea, heat to increase the temperature, when the temperature rises to 120-133°c, keep the reaction for 1-45 minutes, and the reaction is completed , cool to room temperature to obtain phthalimide product;
(2) preparation of 4-nitrophthalimide: take 100-140ml concentrated sulfuric acid, place it in a reaction bottle, add 24ml fuming nitric acid, place it in an ice bath, and cool to 3-8 ℃, add 20g phthalimide with stirring at a rotation speed of 500-1000r/min, continue stirring the reaction for 4h at 10-15℃, then leave it at room temperature for 12-18h, and pour the reaction solution into 0.45-0.8 kg crushed ice, carry out suction filtration at a pressure of 5-15kpa to obtain: product;
(3) preparation of 4-nitrophthalimide: in a container, add 12g 4-nitrophthalimide, 4g hydrazine hydrate, 80ml water, and reflux at 115-130°c after 1-2 hours, an orange-red solution is obtained. after cooling, adjust the ph to 3 with glacial acetic acid to precipitate a large amount of precipitate. wash the precipitate with dilute acetic acid, filter it with suction, and dry it to obtain a light yellow solid product;
(4) preparation of 4-aminophthalic hydrazide (isoluminol): warmly dissolve 12.6g of 4-nitrophthalic hydrazide in 125 ml of 5% sodium hydroxide solution. with constant stirring, drop the solution into a pure solution containing 56g of concentrated stannous chloride hydrochloric acid. add 2-5g of nano-titanium dioxide to the solution. under the irradiation of ultraviolet light, react at 20-30°c for 10-15 minutes to obtain a non-toxic solution. color clear solution, cool to 0-minus 5℃, and get a large amount of white: flocculent precipitate, suction filtration, dissolve the precipitate with 10% ammonia water, filter out the insoluble matter, acidify the filtrate with 20% acetic acid to get a white precipitate, this precipitate is treated with 2% ammonia water dissolve naoh, filter with suction, acidify the filtrate with 20% acetic acid to obtain a white precipitate, filter with suction, and dry to obtain a white powdery solid, which is the isoluminol product;
method 2: a method for preparing isoluminol using phthaloyl hydrazide as raw material, including the following steps:
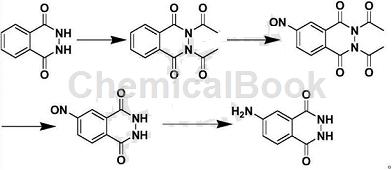
step 1: preparation of diacetyl phthaloyl hydrazide
while stirring at room temperature, add 60g of dry phthaloyl hydrazide waste (47% purity) and 200ml of acetic acid into a 500ml three-necked flask, and stir evenly. add 30g of sodium acetate and stir at room temperature for 30 minutes. then add 40g of acetyl chloride dropwise and finish dropping within 30 minutes. stir at room temperature for 1 hour, then raise the temperature to 70°c and continue the reaction for 5 hours. check the plate to confirm that phthaloyl hydrazide has been completely converted into the desired product, stop the reaction, and allow the reaction solution to naturally cool to room temperature. the reaction solution was directly subjected to the next reaction without further treatment.
step 2: preparation of nitrosodiacetylphthaloylhydrazide
at room temperature, add 15g sodium nitrite in batches to the reaction solution in the previous step. after the addition is complete, stir at room temperature for 3 hours. check with a spot plate to confirm that diacetyl phthaloyl hydrazide has been completely converted into the desired product. after stopping the reaction, acetic acid is recovered by distillation under reduced pressure, and the residue is the crude product of 4-nitrosodiacetylphthaloylhydrazide. this crude product does not require further treatment and can be directly used in the next step of the reaction.
step 3: preparation of nitrosophthaloyl hydrazide
under stirring at room temperature, add the crude 4-nitrosodiacetylphthaloylhydrazide obtained in the previous step to a 500ml three-necked flask, add 200ml of 95v/v% ethanol to it, stir well, and divide into batches add 10g sodium hydroxide. after the addition, reflux the reaction for 5 hours. check the plate to confirm that 4-nitrosodiacetylphthaloylhydrazide has been completely converted into the desired product. stop the reaction and allow the reaction solution to naturally cool to room temperature. the reaction solution was directly subjected to the next reaction without further treatment.
step 4: preparation of isoluminol
while stirring at room temperature, add 10g of sodium thiosulfate at one time to the reaction solution in the previous step. after the addition is complete, stir at room temperature for 30 minutes. then heat and reflux the reaction for 3 hours. check the plate to confirm that 4-nitrosophthalic hydrazide has been completely converted into the required product. stop the reaction and let the reaction solution naturally cool to room temperature. then filter it and collect the filter cake. the filtrate is rotary evaporated to recover 95% ethanol. the residue is combined with the filter cake, added to 200 ml of water, heated to 80°c, and stirred for 30 minutes.�there is still some solid that is insoluble, so filter it while it is hot. add concentrated hydrochloric acid to the filtrate to adjust the ph to 9, and allow natural cooling to crystallize. filter to obtain 26g of light yellow powdery product.
main reference materials
[1] cn201510983063.8 a method for preparing isoluminol using phthaloyl hydrazide as raw material
[2] cn201710051577.9 a method of synthesizing luminol or isoluminol using one-pot method
[3] cn201610458243.9 preparation method of high-yield isoluminol luminescent reagent

 微信扫一扫打赏
微信扫一扫打赏

