Background and overview[1]
2,6-Difluoro-3-methoxyphenylboronic acid can be used as a pharmaceutical synthesis intermediate. If 2,6-difluoro-3-methoxyphenylboronic acid is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and rinse skin thoroughly with soap and water. If discomfort occurs , seek medical attention; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation [1]
2,6-Difluoro-3-methoxyphenylboronic acid can be used as a pharmaceutical synthesis intermediate. For example, prepare the following compounds:
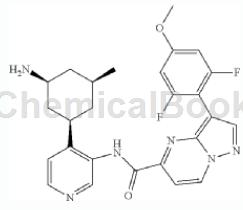
Use tert-butyl[(1S,3R,5S)-3-(3-{[(3-bromopyrazolo[1,5-a]pyrimidin-5-yl)carbonyl]amino}pyridine-4 -5-methylcyclohexylcarbamate and 2,6-difluoro-3-methoxyphenylboronic acid are used as raw materials.
The specific steps are: tert-butyl [(1S, 3R, 5S)-3-(3-{[(3-bromopyrazolo[1,5-a]pyrimidin-5-yl)carbonyl]amino} Pyridin-4-yl)-5-methylcyclohexylcarbamate, 2,6-difluoro-3-methoxyphenylboronic acid (20.4 mg, 0.0756 mmol), dicyclohexyl (2′, 4′, 6′-Triisopropylbiphenyl-2-yl)phosphine-(2′-aminobiphenyl-2-yl)(chloro)palladium (1:1) (3.3 mg, 0.0042 mmol) Tripotassium hydrogen phosphate hydrated A solution of the compound (23.9 mg, 0.104 mmol) in 1,4-dioxane (0.51 mL)/water (0.17 mL) was stirred at 80°C under a N2 atmosphere for 1.5 hours. The reaction mixture was diluted with DCM and washed with water. The organic layer was concentrated under reduced pressure. The residue was purified by preparative HPLC (XBridge™ C18 column, eluting with a gradient of MeCN/water containing 0.05% TFA at a flow rate of 60 mL/min) to afford the Boc protected intermediate. Compounds were treated with 1:1 DCM/TFA (2 mL) for 2 h. The volatile solvent was removed under reduced pressure and the resulting residue was dissolved in MeOH and passed through a preparative LCMS (XBridge™ C18 column, eluting with a gradient of MeCN/water containing 0.1% ammonium hydroxide at a flow rate of 60 mL/min )purification. The title compound was obtained as a white solid (4.3 mg, 18%).
Main reference materials
[1] US20160009726 Bicyclic heteroaromatic carboxamide compounds useful as Pim kinase inhibitors

 微信扫一扫打赏
微信扫一扫打赏

