Background and overview[1]
2-(2-Methoxyphenoxy)malonate dimethyl ester can be used as a pharmaceutical synthesis intermediate. If 2-(2-methoxyphenoxy)malonate dimethyl ester is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and rinse skin thoroughly with soap and water. If you feel any discomfort, seek medical attention; if contact with eyes occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Structure
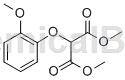
Preparation [1]
The preparation of 2-(2-methoxyphenoxy)malonate dimethyl ester is as follows: Dissolve 2-methoxyphenol (100g, 805.54mmol) in toluene (700ml) at 20-30℃ and add sodium hydroxide (33.83g, 845.75mmol). The reaction mass is heated to reflux temperature and the water is azeotropically separated. After this time, dimethyl chloromalonate (147.6 g, 886.16 mmol) was added at 60-65°C over 30 minutes and heated to reflux temperature and stirred for 3 hours. The reaction mass was cooled to room temperature, washed with DM water, then 1% w/v aqueous sodium hydroxide solution and concentrated to obtain 195 g of the title compound 2-(2-methoxyphenoxy)malonate dimethyl ester.
Apply[1]
2-(2-Methoxyphenoxy)malonate dimethyl ester can be used as a pharmaceutical synthesis intermediate, such as the preparation of 5-(2-methoxyphenoxy)-2-(pyrimidine-2 -base)-ectohydropyrimidine-4,6-dione:
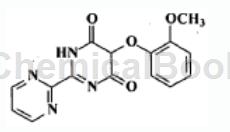
The specific steps are: add 2-(2-methoxyphenoxy)malonate dimethyl ester to the reaction material containing 2-aminopyrimidine hydrochloride at 0-5°C. Sodium ethoxide (106.76g, 1570mmol) was added to the reaction mass at 0-5°C for 1 hour and maintained at 35°C for 5 hours. After that, the reaction mass was concentrated, dissolved in a mixture of DM water (500 ml) and toluene (150 ml), and acidified to pH 0.5-0.7 with dilute hydrochloric acid at 20-30°C. The precipitated product was filtered, washed with toluene, then water, and dried at 75-80°C to obtain 129.3 g of the title compound. Chromatographic purity 96.61% (HPLC, normalized by area).
Main reference materials
[1] WO2011024056 AN IMPROVED PROCESS FOR THE PREPARATION OF BOSENTAN

 微信扫一扫打赏
微信扫一扫打赏

