Background and overview[1]
Methyl 5-bromo-2-nitrobenzoate can be used as a pharmaceutical synthesis intermediate, such as the preparation of benzydamine impurity B. Benzydamine, whose chemical name is 1-benzyl-3-[3-(dimethylamino)propoxy]-1H-indazole, is a non-steroidal anti-inflammatory drug commonly used in surgery and Various inflammations caused by trauma, as well as arthritis, tracheitis, pharyngitis, etc., can be used in combination with antibiotics or sulfonamides; benzydamine’s mouthwash or spray forms are used to treat inflammation of the mouth and throat, and also have local anesthesia. Benzydamine is a cholecystokinin receptor antagonist with anti-cancer properties, especially colon cancer, pancreatic cancer and brain cancer. It also has analgesic and anxiolytic effects. This drug has broad application prospects.
Structure
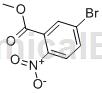
Preparation[1]
The preparation of methyl 5-bromo-2-nitrobenzoate is as follows: Dissolve 36.23g 5-bromo-2-nitrobenzoic acid and 41.72g potassium carbonate in N, N-dimethylformamide, and then Add 61.24g methyl iodide, stir at room temperature for 20 hours, then add 600mL water and 2L ethyl acetate for extraction, dry over sodium sulfate, and concentrate under reduced pressure to obtain a crude product, which is separated and purified by column chromatography to obtain 34.6g of yellow solid compound 5-bromo-2. – Methyl nitrobenzoate, product yield 90.3%.
Apply[1]
Methyl 5-bromo-2-nitrobenzoate can be used as a pharmaceutical synthesis intermediate. For example, the intermediate compound V for the synthesis of benzydamine impurity B:
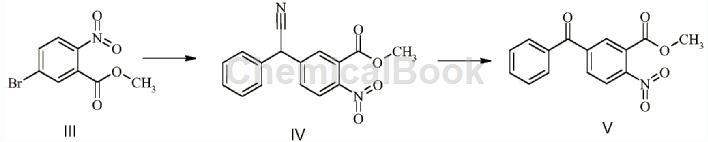
The specific steps are: Dissolve 34g of methyl 5-bromo-2-nitrobenzoate and 23g of phenylacetonitrile in N,N-dimethylformamide, add 29.61g of potassium tert-butoxide under ice bath and stir at room temperature. After 3 hours, compound IV is generated without separation. Add 44.5 mL of 30% hydrogen peroxide to the reaction solution in an ice bath and stir at room temperature overnight. Extract with ethyl acetate, dry over sodium sulfate, and concentrate under reduced pressure to obtain a crude product, which is then separated by column chromatography. 16.3g of intermediate V, yield 50%.
Main reference materials
[1] CN105884687 A kind of preparation method of 5-benzyl benzydamine

 微信扫一扫打赏
微信扫一扫打赏

