Background and overview[1]
5-Bromo-2-chloro-4-fluoroanisole is an ether derivative that can be used as a pharmaceutical synthesis intermediate. If 5-bromo-2-chloro-4-fluoroanisole is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and rinse the skin thoroughly with soap and water. If discomfort occurs , seek medical attention; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
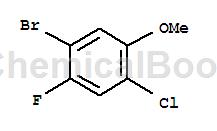
Structure
Preparation[1]
The preparation of 5-bromo-2-chloro-4-fluorobenzole is as follows: add 2-chloro-4-fluorobenzole to a 0.5L magnetically stirred reaction flask equipped with a thermometer, addition funnel and reflux condenser. Ether (112g, 0.7mol) and ZnBr2 (265.4g, 0.92mol, 78%, aq.). The mixture was warmed to 50°C and bromine (112 g, 0.7 mol) was added over 2 hours. After another 2 hours at 54°C, HPLC analysis showed 3.4% CFA, 78.8% 5-bromo-2-chloro-4-fluoroanisole, 12.9% 6-bromo-2-chloro-4-fluoroanisole, and Various unknown impurities. After cooling the mixture to 25°C, a light colored solid precipitated.
Separate it from the mother liquor by filtration, and then wash it three times with 38% NaHSO3 aqueous solution (50ml per wash). At this time, the KI paper test showed that there were no oxidizing species in the filter cake. The filter cake was then washed further three times with water (50 ml per wash) and then dried under vacuum at 50-60°C for 6 hours. The isolated weight of the product was 80 g (HPLC purity 99% pure 5-bromo-2-chloro-4-fluoroanisole, m.p.: 72.2-73.2 °C, containing 18 ppm Zn++ by atomic absorption spectrometry). The mother liquor from the first filtration is collected separately from further washing: it consists of two phases, both bromine-colored. After cooling overnight at 5 °C, a second batch of product was collected by filtration: after washing as described above and drying under vacuum, the product weighed 9.5 g (98% pure 5-BCFA by HPLC). Therefore, the total weight of the isolated product was 89.5 g (yield 53.4%).
Separate the ZnBr2 filtrate from the remaining organic phase (40g), and then extract it three times with MC (15ml each time). The separated bromo-colored MC phase was combined with the above organic phase, decolorized with bisulfite and evaporated to a semi-solid material (49 g). After cooling the mixture overnight, a third batch of product was isolated (4.2 g of 5-BCFA, 94.7% pure by HPLC). Another batch of solids was isolated from this final mother liquor (2.5 g, 74.7% 6-bromo-2-chloro-4-fluoroanisole, 23% 5-bromo-2-chloro-4-fluoroanisole) .
Main reference materials
[1]WO1999019275PROCESFORELECTROPHILICAROMATICSUBSTITUTION

 微信扫一扫打赏
微信扫一扫打赏

