Background and overview[1]
3,5-Diiodo-4-hydroxybenzaldehyde can be used as a pharmaceutical synthesis intermediate. If 3,5-diiodo-4-hydroxybenzaldehyde is inhaled, move the patient to fresh air; if skin contact occurs, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel unwell. ; If eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Structure
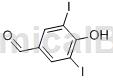
Preparation[1]
The preparation of 3,5-diiodo-4-hydroxybenzaldehyde is as follows: In a 100mL round-bottomed flask, add 4-hydroxybenzaldehyde (1.0g, 8.19mmol), sodium periodate (1.75g, 8.19mmol) ) and NaCl (957 mg, 16.38 mmol) were dissolved in acetic acid (30 mL). Add H2O (3 mL) at room temperature. The reaction mixture was stirred at room temperature for 10 minutes, then potassium iodide (2.72 g, 16.4 mmol) was added and the reaction mixture was stirred at room temperature for 96 hours.
The reaction mixture was diluted with EtOAc (25 mL) and 1 M aqueous sodium thiosulfate solution. The solution (25 mL) was added and the resulting mixture was stirred for 15 minutes. The solution was poured into 250 mL Erlenmeyer and washed with EtOAc (50 mL) and 1M aqueous sodium thiosulfate solution. solution (50 mL) and the resulting solution was stirred for an additional 15 min. The organic layer was separated, and the aqueous phase was further extracted with EtOAc (3 × 50 mL). The combined organic layers were washed with brine (100 mL), dried over Na2SO4, filtered and concentrated to dryness to give 4-hydroxy-3,5-diiodobenzaldehyde (2.91 g, 95% yield) as a light yellow solid .
Apply[1]
3,5-Diiodo-4-hydroxybenzaldehyde can be used as a pharmaceutical synthesis intermediate. For example, prepare the following compounds:
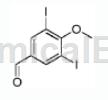
The specific steps are as follows: In a 100mL round-bottomed flask, dissolve 4-hydroxy-3,5-diiodobenzaldehyde (1.0g, 2.67mmol) in acetone (15mL), and add K2CO3 (553mg) at room temperature , 4.0mmol). The mixture was stirred at room temperature for 30 minutes, then methyl iodide (758 mg, 5.34 mmol) was added and the reaction was stirred at reflux for 3.5 hours.
After this time, methyl iodide (2 equiv, 758 mg, 5.34 mmol) was added and the mixture was stirred at reflux for a further 1.5 hours. The acetone was evaporated and the residue was collected in EtOAc (40 mL) and water (60 mL). The aqueous layer was further extracted with EtOAc (3 x 40 mL). The combined organic layers were washed with brine (60 mL), dried over Na2SO4, filtered and concentrated to dryness to give a yellow oil. The oil was purified by column chromatography (SiO2, eluent: cyclohexane: EtOAc = 100:0 to 90:10), evaporated and dried to give 3,5-diiodo-4-methoxybenzaldehyde (493 mg , 48% yield) as a white solid.
Main reference materials
[1] WO2011151423 SUBSTITUTED ISOQUINOLINES AND THEIR USE AS TUBULIN POLYMERIZATION INHIBITORS

 微信扫一扫打赏
微信扫一扫打赏

